Abstract
BACKGROUND AND PURPOSE: Reproducible animal models facilitate preclinical assessment of aneurysm therapies. Our purpose was to determine if increased elastase doses enlarge aneurysms and parent arteries.
METHODS: Rabbit right common carotid artery (CCA) aneurysms were created with distal ligation and intraluminal elastase incubation. Groups were 1) sham (no elastase, n = 3), 2) 25% elastase (10 minutes, n = 9), 3) 50% elastase (10 minutes, n = 7), and 4) 50% elastase (20 minutes, n = 41). Angiography was performed after 14 days. Resultant aneurysm width and height and parent artery diameters were measured and compared with the Student t or Mann-Whitney (Wilcoxon rank sum) test.
RESULTS: Proximal segments were enlarged in all elastase subjects and no sham subjects. Mean measurements were significantly smaller in the sham group than in other groups. Aneurysm widths and heights, respectively, were 3.8 mm ± 0.8 and 7.4 mm ± 2.0 in the low-dose group; 3.9 mm ± 1.3 and 8.5 mm ± 3.8, medium-dose group; and 4.1 mm ± 1.1 and 8.7 mm ± 2.6, high-dose group. Differences were not significant. Parent artery widths were 3.5 mm ± 0.7, 3.8 mm ± 0.7, and 4.3 mm ± 1.4 in the low-, medium-, and high-dose groups, respectively; the high-dose group had larger arteries (P = .07).
CONCLUSION: Aneurysms were reliably created and were sized similar to human intracranial aneurysms. Elastase concentration and incubation duration did not affect resultant size. Relatively short incubation (eg, 10 minutes) and 25% elastase can be used to create rabbit aneurysms, especially if dilatation of adjacent parent arteries is to be avoided.
Preclinical assessment of emerging aneurysm therapies is greatly facilitated by reproducible animal models. Numerous species of animals, including rat, rabbit, canine, swine, sheep, and primate species, have been proposed as valid model systems. Both arterial aneurysms and vein pouch aneurysms have been proposed. Some models require exquisite microsurgical skills (1), whereas others rely on simple surgeries, such as vessel ligation, alone (2–5).
A simple model of arterial saccular aneurysm created in rabbits has been reported previously (3, 4). This technique involves ligation of the common carotid artery (CCA) and elastase treatment of the vessel wall. Aneurysms are reliably created, even by operators with little or no surgical experience. Resultant aneurysm cavities simulate the size and shape of human intracranial aneurysms, and parent artery models simulate the tortuosity and diameter of human intracranial vessels.
Although our previously reported rabbit model reliably results in aneurysm formation (2–5), we hypothesized that the size of the aneurysm cavity and the diameter of the adjacent parent artery is influenced by the degree of elastase-induced vessel injury. In the current article, we report the results of a dose-escalation study to determine whether an increasing dose of elastase, measured in terms of elastase concentration and incubation time, results in larger aneurysms and increased diameters in the adjacent parent arteries.
Methods
Detailed procedures for aneurysm creation have been described elsewhere (2–5). Briefly, New Zealand White rabbits (3–4 kg) were anesthetized with ketamine and xylazine (60 and 6 mg/kg, respectively). With a sterile technique, the right CCA was exposed and ligated distally. A 5F sheath (Cordis Endovascular, Miami Lakes, FL) was advanced in a retrograde fashion in the CCA to a point approximately 3 cm cephalic to the CCA origin. Through this indwelling sheath, a 3F Fogarty balloon (Baxter Healthcare Corporation, Irvine, CA) was advanced to the origin of the right CCA at its junction with the subclavian artery. The balloon was inflated with just enough iodinated contrast material to arrest flow in the CCA. Porcine elastase mixed with saline and iodinated contrast material was incubated in the dead space of the CCA above the inflated balloon. After incubation of the elastase solution, the balloon and sheath were removed, and the CCA was ligated below the sheath entry site.
Previous experience demonstrated that resultant saccular dilatation of the CCA origin ranges from 3 to 10 mm in diameter and from 6 to 12 mm in height (5). In addition, the diameter of the adjacent brachiocephalic and subclavian arteries proximal and distal to the CCA origin may vary in diameter after the procedure, ranging from 2.5 to 4.5 mm in diameter. These dimensions result in moderate variability in aneurysm morphology and neck width.
The degree of elastase-induced injury may be related to both the concentration of elastase and the duration of elastase incubation. Previous publications have reported the use of porcine elastase (5.23 units per milligram, 40.1 mg/mL; Worthington Biochemical Corporation, Lakewood, NJ); by volume, 50% was mixed with 25% normal saline and 25% iodinated contrast material (Omnipaque 300; Nycomed, Princeton, NJ). This mixture was infused into the artery lumen and allowed to dwell there for 20 minutes. In our opinion, this incubation duration and concentration are at the upper limits of what we consider practical for this procedure. For the current study, we designated this dose of elastase (50% elastase by volume incubated for 20 minutes) as the high dose. In addition, we studied three other doses: 1) sham, 50% contrast material and 50% saline incubated for 20 minutes; 2) low, 25% elastase with 50% contrast material and 25% saline incubated for 10 minutes; and 3) medium, or 50% elastase with 25% contrast material and 25% saline incubated for 10 minutes. The four dose groups are shown in the Table. Because the high-dose group represents our current standard dose, we included 41 consecutive subjects in this group. The sham group included three subjects, the low-dose included nine subjects, and the medium-dose group included seven subjects.
Four dose groups
All subjects were imaged at least 21 days after aneurysm creation; this timing was based on our previous data demonstrating that the aneurysm cavities may enlarge for as long as 21 days after their creation. Animals were anesthetized as before. A 24-gauge angiocatheter was placed in a right or left ear vein. During digital subtraction imaging at a rate of two frames per second, 5 mL of iodinated contrast medium (Omnipaque 300; Nycomed) was infused into the ear vein. Imaging was performed during the arterial phase. An external sizing device was in place during intravenous digital subtraction angiography. A single observer (N.H.F.) measured the width and height of the aneurysm cavities, which were determined in reference to the external sizing device. The width of the aneurysm cavity was determined at its point of maximum width, and the height was measured from the dome to the line connecting the proximal and distal aneurysm neck. The diameter of the adjacent parent artery immediately proximal to the origin of the aneurysm cavity was also measured. All measurements were compared between each dose group by using the Student t test or, when appropriate, the Mann-Whitney (Wilcoxon rank sum) test.
Results
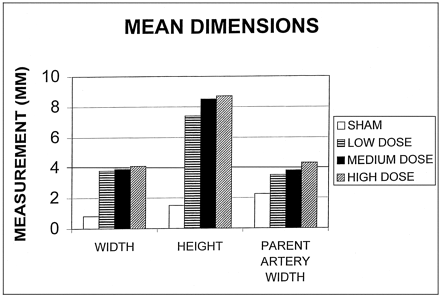
A summary of the mean dimensions is shown in Figure 1. Representative images are shown in Figure 2.
Bar graph shows a summary of the mean dimensions.
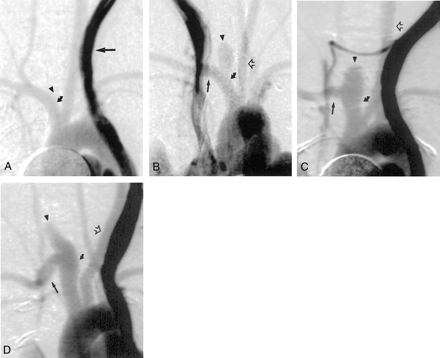
Intravenous digital subtraction angiograms, anteroposterior views, obtained in animals from each group.
A, Sham group, 4 weeks after surgery. No aneurysm cavity is present at the stump of the right CCA (arrowhead). The parent artery (curved arrow) is not significantly dilated. Contrast enhancement within the persistent left superior vena cava is darker than arterial contrast enhancement because of stasis in the venous structure (straight arrow).
B–D, Low-dose (25% elastase, 10 minutes) (B), medium-dose (50% elastase, 10 minutes) (C), and high-dose (50% elastase, 10 minutes) (D) groups, 21 days after surgery. Brachiocephalic arteries are opacified, including the left CCA (open arrow); right subclavian artery (straight solid arrow); and aneurysm cavity, ie, aneurysmal dilatation of the right CCA origin (arrowhead). In B, the parent artery (curved solid arrow) is not dilated. In C, the parent artery (curved solid arrow) is slightly dilated compared with that in A and B. In D, the proximal and distal parent arteries (curved solid arrow) are remarkably dilated compared with those in A and B.
Aneurysm Width
Mean widths for the sham group and low-, medium-, and high-dose groups were 0.8, 3.8, 3.9, and 4.1 mm, respectively. The width of the sham group was significantly smaller than that of all other groups. However, no difference between any of the three groups undergoing elastase injury was statistically significant.
Aneurysm Height
Mean heights for the sham group and the low-, medium-, and high-dose groups were 1.5, 7.4, 8.5, and 8.7 mm, respectively. The height in the sham group was significantly smaller than that of all other groups. The difference in the height of aneurysm cavity among the other three groups was not statistically significant; although, compared with the low-dose group, the high-dose group had a trend toward taller aneurysms (P = .15, Mann-Whitney [Wilcoxon rank sum] test).
Parent Artery Width
The mean width of the parent artery adjacent to the aneurysm were 2.5, 3.5, 3.8, and 4.3 mm, respectively, for the sham group and low-, medium-, and high-dose elastase injury groups. The mean diameter of the sham group was significantly smaller than that of all other groups. Compared with the low-dose group, the high-dose group had a trend toward larger-diameter parent arteries (P = .07).
Discussion
These findings demonstrate that dilated saccular arterial cavities form at the origin of the right CCA in New Zealand White rabbits after intraluminal incubation of various concentrations of porcine elastase but that arteries subjected to identical surgical procedures without elastase fail to form dilated cavities. We further determined that increasing elastase injury, defined as increased elastase concentration, duration of incubation, or both, failed to result in larger aneurysm cavities. These data indicate that short-duration incubation of dilute elastase achieves sufficient disruption of elastin to allow aneurysmal enlargement in the artery remnant.
A well-accepted tenet of medical radiology is that occlusion of an artery results in retrograde thrombosis in that same artery to the site of any major branch vessel. For instance, occlusion of the internal carotid artery (ICA) in its midcervical region results in thrombosis of the ICA extending to the carotid bulb. The sham group in our study demonstrated this phenomenon, with near obliteration of the arterial stumps in all three sham cases. Thus, elastase injury is considered crucial to the induction of aneurysm dilation and the persistent patency of arterial stumps in this animal model. To our surprise, increasing the dose and duration of elastase injury failed to alter aneurysm width or height; this result indicated that even mild elastase injury suffices for arterial dilation.
Although the final common pathway of the elastase injury consists of the degradation of the elastic lamellae, the pathophysiologic mechanisms leading to thinned lamellae likely are multifactorial. Inflammation has been a prominent feature in elastase-induced abdominal aortic aneurysms in rats (6). Other studies of the rat abdominal aortic aneurysm model have implicated matrix metalloproteases (MMPs) as the key elements in the degradation of elastin (7–12). Inflammatory cells elicited by the elastase injury may express these proteases, which serve to degrade multiple extracellular matrix proteins. Our group is currently studying the expression of MMPs in our rabbit model.
On the basis of extensive practical experience with our model in developing and testing novel devices for aneurysm occlusion, we have occasionally noted marked dilatation of the brachiocephalic and subclavian arteries adjacent to the aneurysm cavities. This marked enlargement can severely hinder attempts at intrasaccular embolization because of the resultant wide neck. We consider such parent artery dilatation unfavorable. We acknowledge that other investigators might welcome the opportunity to study fusiform dilatation in the brachiocephalic artery in rabbits. The precise mechanism with which the parent artery becomes dilated is unknown, but dilatation may result from subtle leakage of elastase solutions during incubation, although the solution was opacified with contrast material, and we were careful to prevent such leakage. We hoped that the dose-escalation study would allow us to choose a dose of elastase that allowed aneurysm formation without parent artery dilatation. Although the difference in the diameters of the parent arteries between any two elastase-injury groups was not statistically significant, the high-dose group had a strong trend toward smaller parent arteries, compared with the low-dose group. These results have caused us to replace our standard dose and duration of elastase with the low-dose regimen.
Conclusion
The concentration of elastase and the duration of incubation had no effect on the size of the resultant aneurysms. However, a relatively short incubation on the order of 10 minutes and a 25% elastase concentration can be used to create model aneurysms in rabbits, especially if dilatation of the adjacent parent artery is to be avoided.
References
- Received November 18, 2000.
- Accepted after revision January 23, 2001.
- Copyright © American Society of Neuroradiology