Abstract
Summary: We report serial CNS findings in a girl with sickle cell disease and stroke. Religious considerations precluded transfusion and bone marrow transplantation; therefore, she received single-agent hydroxyurea therapy for almost 6 years. MR angiography showed that vascular patency improved, although diffuse cerebral atrophy slowly worsened. Hydroxyurea can be effective in treating vasculopathy, but it might not prevent the progression of parenchymal damage in advanced disease.
Damage to the brain is a common consequence of sickle cell disease (SCD), even in very young children (1, 2). Roughly 11% of children with the most serious form of SCD (hemoglobin SS) have a stroke by age 20 years, usually by the end of the first decade (3). The underlying etiology is thought to be an infarctive event secondary to large-vessel stenosis or occlusion (4). Bone marrow transplantation can potentially reverse the vasculopathy common in SCD (5), but whether this or any other treatment can halt or reverse damage to the brain parenchyma is not yet known.
Treatment with hydroxyurea is known to increase the production of hemoglobin F (Hb F), to reduce total white blood cell count and absolute neutrophil count, and to improve erythrocyte rheological characteristics (6, 7). Recent evidence suggests that hydroxyurea can also ameliorate the clinical severity of SCD in children (7). Nevertheless, the use of hydroxyurea in children is controversial; although the toxicity of the drug appears to be mild, transient, and reversible (8), the chronic effects are not well known. Hydroxyurea could potentially be leukemogenic, if given for a prolonged period (9). Furthermore, the response to hydroxyurea is unpredictable, and the reversibility of end-organ damage has not been established (10). Preliminary evidence also suggests that, after the discontinuation of transfusion therapy, hydroxyurea alone may not be able to prevent recurrent stroke (7). In this context, determining whether treatment with hydroxyurea can have a beneficial effect on the brain is critically important.
Case Report
In 1996, a 7-year-old African-American girl with SCD (hemoglobin SS) presented with new-onset left hemiparesis. On examination, she had partial paralysis of her left arm, an ataxic gait, and difficulty with ambulation. MR imaging showed a recent stroke in the region of the right middle cerebral artery territory, as well as a remote left anterior cerebral artery infarct, bilateral deep white matter leukoencephalopathy, and a few scattered deep white matter lacunae (Fig 1 A and B). The patient was enrolled into a local trial of hydroxyurea based on the HUG-KIDS Phase I/II study (8). Treatment was begun at a dose of 15 mg/kg by mouth daily, with dose escalation (by 5 mg/kg every 8 weeks to a maximum of 30 mg/kg/d) if no toxicity was observed. Serial hematologic evaluations were performed every 2 weeks to monitor for toxicity (Fig 2).
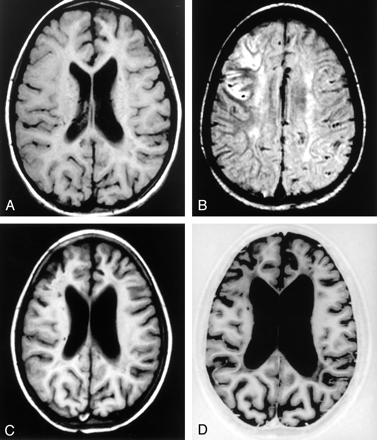
Serial MR images in a patient receiving hydroxyurea. The first examination was performed at another institution.
A, Examination 1. T1-weighted image shows mild atrophy in the left medial frontal lobe.
B, Examination 1. Fluid-attenuated inversions recovery image demonstrates a recent infarction in the right middle cerebral artery territory, as well as a remote left anterior cerebral artery infarct, bilateral deep white matter leukoencephalopathy, and a few scattered deep white matter lacunae.
C, Examination 2. Image obtained at our institution almost 1 year after the beginning of therapy shows further progression of mild diffuse cerebral atrophy.
D, Examination 5. Approximately 4 years after the beginning of hydroxyurea therapy moderate diffuse cerebral atrophy is present, with prominent sulci and further ex vacuo dilatation of the ventricular system.
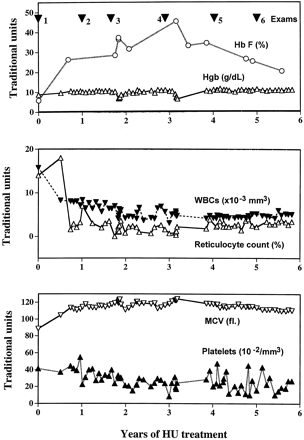
Summary of the clinical history of the patient. Plots show the MR imaging dates, with the percentage of fetal hemoglobin (Hb F), hemoglobin (Hgb), white blood cell (WBC) count, reticulocyte count, mean corpuscular volume (MCV), and platelet count after the initiation hydroxyurea treatment at time 0. HU indicates hydroxyurea.
The patient had a second stroke 1 month after beginning hydroxyurea therapy. CT examination of her head revealed extension of the right frontoparietal infarct. She was treated for 1 month in an out-patient rehabilitation facility. She was also hospitalized for a pain crisis 2 months after beginning hydroxyurea treatment and for an episode of acute chest syndrome 3 months after beginning therapy.
The patient received a neurology consultation 8 months after beginning hydroxyurea therapy. She was seen for an expressive language problem, occasional staring spells, and a recent decline in school performance. She was noted to have mild left hemiparesis and right exophoria, left-sided hyper-reflexia, and a mild delay in answering questions. EEG revealed a right frontocentral region spike-wave abnormality, and valproic acid (250 mg by mouth twice daily) was initiated to treat complex partial seizures. This agent was preferred because it has been noted to up-regulate Hb F (11). Subsequent neurologic examinations demonstrated subtle but stable bilateral hemiparesis and hyper-reflexia (left greater than right), and a persistent wide-based gait. Valproic acid was continued, but an increased dose was required to control seizures.
One year after beginning treatment, the patient underwent routine evaluation at our institution (examination 2, Fig 2), with MR imaging (Fig 1C) and MR angiography (Fig 3A and B). The MRA was optimized for a high rate of blood flow by using the following parameters: TR/TE, 34/5; field of view, 20 cm; flip angle, 20°; matrix, 192 × 256; and effective section thickness, 1 mm. Subsequent MRA images were further optimized for high rate of blood flow with these parameters: TR/TE, 24.5/4.8; field of view, 20 cm; flip angle, 25°; matrix, 192 × 512; and effective section thickness, 1 mm. Bifrontal encephalomalacia was seen, greater on the right than on the left (Fig 1C), with multiple lacunae in the deep white matter, leukoencephalopathy, and mild ex vacuo dilatation of the ventricular system. Vasculopathy was severe (Fig 3A and B), and small moyamoya collaterals were present near the right A-1 and M-1 segments. Eight months later (examination 3, Fig 3 C and D), with the MRA examination further optimized for rapid flow, vasculopathy had generally improved, but moyamoya was still present.
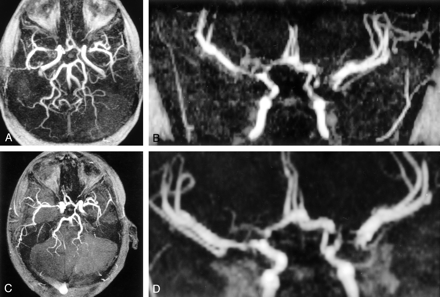
Serial MRA images in a patient receiving hydroxyurea. Images show progressive improvement in vascular patency.
A and B, Examination 2. One year after beginning therapy, an 8-mm severe stenosis is present in the left ICA, with a large left posterior communicating (PCOM) artery. Also present is a 10-mm stenosis in the proximal right A-1 segment of the ACA, as well as proximal segmental stenoses in both middle cerebral arteries (MCAs) (11 mm, right M-1; 9 mm, left M-1). Small moyamoya collaterals are present near the right A-1 and M-1 segments.
C and D, Examination 3. Eight months later, with the MRA examination further optimized for rapid flow, the left ICA stenosis has nearly resolved, with only a 5-mm region of tapering involving the supraclinoid segment. Stenoses involving the left M-1, right A-1, and right M-1 segments had decreased to 7, 3, and 3 mm, respectively. A short 2-mm proximal stenosis is present in the left A-1 segment. Moyamoya vasculopathy was unchanged.
Almost 3 years after beginning therapy, MR imaging (examination 4) showed further progression of mild diffuse cerebral atrophy, but MRA continued to show improvement, with resolved right A-1 and M-1 stenoses and mild moyamoya vasculopathy (Fig 4A and B). The right MCA appeared to be more patent (Fig 4B); small-vessel conspicuity was lost; and both PCOMs had become smaller (Fig 4A), suggesting a slower rate of blood flow (10). Approximately 4 years after beginning hydroxyurea therapy, the patient was examined again (examination 5) with MR imaging (Fig 1D) and MR angiography (Fig 4C and D). Moderate diffuse cerebral atrophy was present, with prominent sulci and further ex vacuo dilatation of the ventricular system (Fig 1D). However, the vasculopathy had continued to improve (Fig 4C and D).
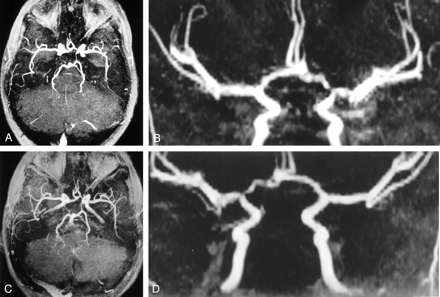
Serial MRA images in the patient in Figure 3. Images show progressive improvement in vascular patency.
A and B, Examination 3. The bilateral A-1 segments have become equivalent in size and the distal left ICA is less tapered. A 2-mm stenosis of the proximal left A-1 and a 1-mm stenosis of the proximal left M-1 segments persist. Also noted are the resolution of the right A-1 and M-1 stenoses and the moyamoya vasculopathy with persistent short-segmental stenoses in the proximal left A-1 and M-1 segments. The right MCA appears to be more patent; small-vessel conspicuity is lost; and both PCOMs have become smaller, suggesting a slower rate of blood flow.
C and D, Examination 4. Persistent short segment stenoses of the proximal left A-1 and M-1 segments remain.
Discussion
We describe considerable improvement in vascular patency in a Jehovah’s Witness who received single-agent chemotherapy with hydroxyurea in lieu of long-term blood transfusion for the management of stroke. Improvement in cerebral vasculopathy has been documented at conventional angiography in patients with SCD who are receiving chronic transfusion (12, 13). We recently described notable improvement in vascular patency, as assessed at MRA, in patients with SCD who underwent bone marrow transplantation (5). However, to our knowledge, this is the first report of improvement in cranial vasculopathy after hydroxyurea treatment.
Although rapid blood flow in patients with SCD can produce flow-dephasing of the signal intensity (which could exaggerate the degree of stenosis despite the use of flow-corrected techniques [14]), we believe that our patient met the criteria for true stenoses (15). Four intracranial vessel segments had initial stenoses longer than 6 mm, with right-sided moyamoya vasculopathy and bihemispheric infarcts. Although we cannot unequivocally demonstrate that vascular patency improved during treatment, we believe that the improvement in patency was nevertheless real. This patient was not evaluated with angiography, which is used less often now than in the past because of the inherent risk in patients who already have an elevated risk of stroke (3, 5, 13). Instead, we used an MRA sequence that was optimized for the conditions of fast blood flow that are typical of children with SCD (5), and the sequence was used without modification after examination 1. Thus, MRA apparently improved in the absence of any technical change. Improvement was especially obvious in the right MCA, which essentially normalized over the first 2 years of therapy (Fig 3A and E). This change in vascular patency is convincing, because moyamoya vessels near the right MCA disappeared entirely during treatment (Figs 3B and D, 4B and D). Unfortunately, despite this improvement in vascular patency, concurrent progression of parenchymal atrophy occurred in our patient. We note that this patient had acute chest syndrome and a poorly controlled seizure disorder, which could also have contributed to her progressive atrophic changes. Such considerations suggest that patients may need to be treated sooner in the course of their disease.
Our findings are consistent with reports that hydroxyurea alone may be unable to prevent the recurrence of stroke, especially in patients with severe preexisting vasculopathy (7, 10). In two reported cases (10), despite %Hb F levels of 20% or more, end-organ damage could progress during hydroxyurea treatment and stroke could occur. Although hydroxyurea may be an alternative to blood transfusion for the prevention of recurrent strokes, patients may be relatively vulnerable to recurrent ischemic stroke 3–4 months after long-term transfusions are ended, before the maximum hematologic protective effects of hydroxyurea therapy begin (7). In addition, our patient had an episode of acute chest syndrome soon after treatment began, and she developed a chronic seizure disorder, both of which may have increased her risk of hypoxic brain injury. Our findings are thus consistent with the presence of a vulnerable period before the beneficial hematologic effects of hydroxyurea therapy occur.
Although the precise mechanisms of the protective effect of hydroxyurea are unknown, the increase in the percentage of Hb F (%Hb F), which increases the fraction of red blood cells with sufficiently long delay times to escape the microcirculation before polymerization can occur (16), presumably helps to prevent sickling in stenotic intracranial vessels (7). Other study findings suggest that a reduction in neutrophils and reticulocytes, an increased total hemoglobin concentration (17), or improved erythrocyte rheological properties (16), may play a role in stroke reduction.
Our patient had an extraordinarily strong response to treatment. In a study of 150 patients with SCD, the response to hydroxyurea was graded by quartiles. In the most responsive quartile, %Hb F increased from 6.4% before treatment to 18.1% after 2 years (18). In our patient, %Hb F improved from 5.9% before treatment to an average of 31.4% after hydroxyurea therapy (Fig 2). The increase in %Hb F in our patient was thus nearly twice as much as the average (18). This effect may have been related to her relatively young age (17). In general %Hb F tends to decrease over time with treatment (18), as in our patient. Nevertheless, over 4 years of treatment, the average %Hb F in our patient increased dramatically, and all of her other blood values tended to normalize (Table). The increase in %Hb F in our patient might have been secondary to valproic acid treatment for the partial complex seizures (11). In any case, the increase in %Hb F and the improved hematologic status did not protect our patient from the progression of parenchymal damage to the brain (Fig 1).
Hematologic values in the case patient before and during treatment with hydroxyurea
Our results show that hydroxyurea can be well tolerated (8), that it can improve hematologic status (8), and that it can reverse damage to the vasculature of the CNS. Hydroxyurea is a treatment alternative that is available to patients who are not candidates for transfusion or bone marrow transplantation. However, hydroxyurea did not halt or reverse the progression of parenchymal damage in our patient, perhaps because advanced disease was present before treatment began.
Acknowledgments
We thank Mary Fitchpatric, Mary Freeman, Crystal Manchester, and Mark Summers, who performed all of the MR imaging.
Footnotes
This work was supported in part by the National Institutes of Health grant HL 60022 (R.G.S.) and by the American Lebanese Syrian Associated Charities.
References
- Received March 11, 2002.
- Accepted after revision July 1, 2002.
- Copyright © American Society of Neuroradiology