Abstract
Summary: We describe a time-resolved, contrast-enhanced carotid MR angiographic technique using a 3D gradient-echo sequence in combination with a sensitivity-encoding scheme. We delineate the steps involved in generating the dynamic MR angiographic images, discuss issues related to image quality, and outline differences between this technique and recently reported similar methods that implement multiple parallel receivers to reduce imaging time.
Advantages of time-resolved, contrast-enhanced 3D carotid MR angiography are the ability to provide information regarding the anatomy and flow dynamics in the neck vessels without the need for postacquisition extraction of carotid arteries from superimposed veins, and contrast bolus timing (1–3). Reducing imaging time to resolve the carotid arteries has, in general, necessitated partial and variable k-space sampling for each dynamic acquisition, view sharing, and/or temporal interpolation (1, 2). These time-resolved MR angiographic techniques are limited by time-demanding image reconstruction algorithms because of variability in k-space sampling (1). They are also susceptible to ghosting, blurring, and reduction in image detail resulting from variability in intravascular concentration of contrast agent among the shared views (4, 5).
Sufficient improvements in imaging speed to time-resolve the carotid arteries can be achieved without the need for view sharing, or temporal interpolation, by implementing sensitivity encoding (SENSE) to complement more traditional magnetic gradient spatial encoding (6, 7).
Using multiple surface coils, each with its own receiver circuit, operating in a phased-array configuration, SENSE permits unfolding of reduced–field of view (FOV) images (ie, images obtained by equal reduction in FOV and number of phase-encoding steps) into full-FOV images with commensurate decreases in imaging time and maintenance of spatial resolution. The reduction in the number of phase-encoding steps is compensated for by spatial encoding based on the location of a signal source with respect to the individual receiver coils.
In this tehnical note, we describe a time-resolved, contrast-enhanced 3D carotid MR angiographic technique combining sensitivity and gradient encoding. We delineate the steps involved in generating the dynamic MR angiographic images, discuss issues related to image quality, and outline differences between this technique and recently reported similar methods that implement multiple parallel receivers to reduce imaging time.
Description of the Technique
Subjects
Institutional review board approval and informed consent from two volunteers (26-year-old woman and 40-year-old man) and two patients (87-year-old woman and 62-year-old man) were obtained before the beginning of the study. Both patients underwent catheter digital subtraction angiography (DSA) on the same day of the MR angiogram. Both patients were suspected of having severe stenoses at the bifurcation of one carotid artery by duplex sonography.
MR Imaging
Carotid MR angiographic studies were performed on a 1.5-T MR system (ACS-NT Compact Plus; Philips Medical Systems, Shelton, CT) with maximum gradient capability of 23 mT·m-1 and slew rate of 103 mT·m-1·ms-1. The imager was operating with software release 6.2 supplemented by a research patch file (SYNCRA 4, Philips Medical Systems, Best, the Netherlands) that provided SENSE capabilities. MR images were obtained with two elliptical phased-array receiver coils (15 cm in diameter) placed on both sides of the neck, and the body coil operating in send-receive mode.
The MR imaging protocol consisted of: 1) coronal 2D phase-contrast MR angiography (TR/TE/flip angle/velocity encoding: 9.5/5.4/15o/70 cm/s) was used to localize the carotid artery bifurcation; 2) full-FOV, low resolution (voxel size: 4.7 × 9.4 × 8 mm3) coronal 3D RF-spoiled gradient-echo MR imaging (TR/TE/flip angle/excitations: 16/4.6/15°/8) of the neck and head was obtained consecutively with the body coil and both parallel receiver coils. The sensitivity maps were calculated by dividing the individual parallel receiver coil images by the body coil image and were refined by a fitting procedure performing noise elimination and sensitivity extrapolation (6); 3) reduced-FOV dynamic coronal 3D RF-spoiled gradient-echo MR imaging of the neck and head was initiated 5 s after the start of a 30 mL gadolinium bolus administered intravenously at a rate of 4 mL/s using a Medrad Spectris power injector (Medrad, Indianola, PA). A total of 10 dynamic images were obtained to track the contrast bolus. The imaging parameters for the dynamic images were varied slightly among subjects because of image quality considerations: TR/TE/flip angle/excitations: 4.3–4.9/2.0–2.4/35°/1, FOV: 250–270 × 60–65 × 50 mm3 (24% rectangular FOV), matrix: 256 × 51 × 21–25 (giving an actual voxel size of 0.98–1.05 × 1.17–1.27 × 2–2.4 mm3 with reconstruction to 0.49–0.53 × 0.49–0.53 × 1–1.2 mm3 after zero filling in all three directions), and read-out bandwidth 440–733 Hz/pixel. Implementing partial (60%) Fourier imaging in the phase-encoding direction for all subjects, the acquisition time per dynamic image varied between 4.0 and 5.9 s, respectively. The protocol was optimized over all subjects based on signal-to-noise ratio (SNR), spatial, and time resolution considerations. The following parameters were found to provide the best compromise between SNR, spatial, and time resolution: TR, 4.9; TE, 2.4; flip angle, 35°; excitation, 1; read-out bandwidth, 440 Hz/pixel; FOV, 270 × 65 × 50 mm3 (24% rectangular FOV) and matrix, 256 × 51 × 21, giving an actual voxel size of 1.05 × 1.27 × 2.4 mm3 with reconstruction to 0.53 × 0.53 × 1.2 mm3 after zero filling in all three directions.
Image processing included unfolding of the aliased image based on the information provided by the sensitivity maps, subtraction of the first volume from the series, and reconstruction of the maximum intensity projection (MIP).
The instances of dynamic acquisitions with pure arterial phase (ie, no venous contamination) were recorded for each of the four subjects. The accuracy of luminal representation (compared with DSA) was assessed for the two patients.
Results
Successful MIPs of both carotids were obtained with the proposed technique in all subjects. At least one dynamic acquisition with a pure arterial phase was present in each of the four subjects, thus obviating postacquisition segmentation.
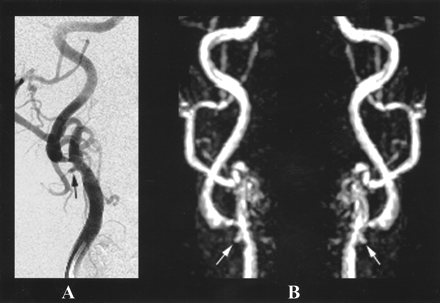
On DSA images, the first patient (87-year-old woman) had severe and mild stenosis in the right and left carotid bifurcations, respectively, while the second patient had severe and mild stenosis in the left and right carotid bifurcations, respectively. The degree of stenosis was similar on MR angiograms and DSA images in both patients (Fig 1).
87-year-old woman with arteriosclerotic disease in both common carotid arteries.
A, Oblique-projection catheter DSA image of the right common carotid artery demonstrates atheromatous plaque at the bifurcation, with ulceration and severe stenosis at the origin of the internal carotid artery.
B, Corresponding MIPs obtained from the contrast-enhanced MR angiogram (TR/TE/flip angle: 4.9/2.4/35°) adequately demonstrate the stenosis and the ulceration.
Discussion
SENSE has been recently implemented to hasten different MR imaging read-out techniques such as spin-echo, fast spin-echo, gradient-echo, and echo-planar (6, 7). In this technical note, SENSE improved the speed of a gradient-echo-based technique by a factor equal to the number of parallel receiver coils used (ie, factor of two). Thus, SENSE provides time-resolved (< 6 s), contrast-enhanced, 3D carotid MR angiography with submillimeter in-plane resolution and adequate coverage that does not require view sharing, or temporal interpolation. In our limited experience with this MR angiographic technique, a pure arterial phase is achieved in all the normal and diseased carotid arteries without the need for bolus timing or postacquisition segmentation.
SENSE MR angiography entails the following steps: 1) acquisition of sensitivity maps before the intravenous administration of contrast agent; 2) acquisition of dynamic reduced-FOV data during the passage of the contrast bolus; 3) reconstruction of reduced-FOV images; and 4) unfolding of reduced-FOV images (7). Further postprocessing steps include 5) subtraction of the first volume from all successive ones and 6) creation of general or targeted MIPs for studying specific parts of the carotid arteries.
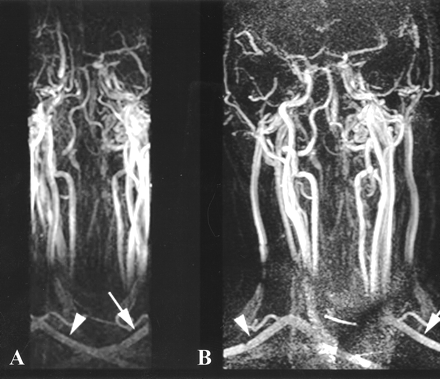
The sensitivity maps are calculated on-line by dividing two full-FOV images, obtained simultaneously with each parallel receiver coil, by a full-FOV reference image obtained with the body coil. These maps are crucial for the SENSE (unfolding) process of the dynamic reduced-FOV images. Each dynamic reduced-FOV data set is acquired simultaneously with both parallel receiver coils. In our study, the FOV and the number of phase-encoding steps in one dimension (ie, in-plane y-direction) are reduced by a factor of two, hence halving the acquisition time of each dynamic image and maintaining spatial resolution, but decreasing SNR. Reconstruction of the reduced-FOV data, using discrete Fourier transform, yields dynamic reduced-FOV images corrupted by aliasing in the y-direction. Finally, the aliased images are unfolded using spatial encoding information from the sensitivity maps. This strong aliasing effect is seen in Figure 2, in which an MIP of the mixed arteriovenous phase of the gadolinium bolus is reconstructed in one of the healthy volunteers before and after SENSE reconstruction. Note that the unfolding process completely removes any kind of aliasing in this case.
26-year-old healthy volunteer.
A and B, MIPs before (A) and after (B) the SENSE reconstruction of the contrast-enhanced MR agiography (TR/TE/flip angle: 4.9/2.4/35°) data. Before SENSE reconstruction, there is aliasing artifact affecting the periphery of the FOV, resulting in overlap of the arteries and veins. After SENSE reconstruction, the aliasing is resolved and the vessels are adequately demonstrated (B). The arrowheads and arrows demonstrate the unfolding of the right and left subclavian arteries, respectively.
It is important to emphasize that with SENSE, the improvement in time resolution is at the expense of SNR. The loss in SNR is at least equivalent to the square root of the reduction in acquisition time. In fact, the SNR in the reconstructed images (SNRSENSE) is: 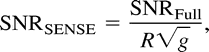 in which R is the FOV reduction factor and g is the “geometry factor,” describing the constructive interaction of noise coming from both elements of the phased-array coil. In our case, the use of two parallel circular coils is almost optimal, rendering g ≈ 1 (6, 7).
in which R is the FOV reduction factor and g is the “geometry factor,” describing the constructive interaction of noise coming from both elements of the phased-array coil. In our case, the use of two parallel circular coils is almost optimal, rendering g ≈ 1 (6, 7).
The mean intraarterial SNR at peak contrast bolus in the first volunteer ranged from 12 in the center of the FOV to 4.5 at the periphery of the FOV. To improve SNR, we reduced the read-out bandwidth by 60% and slightly increased the FOV from 250 mm to 270 mm in the second volunteer. This resulted in a 13% increase in acquisition time of each dynamic image and a 50% increase in SNR. The changes in FOV and read-out bandwidth were maintained for patient imaging.
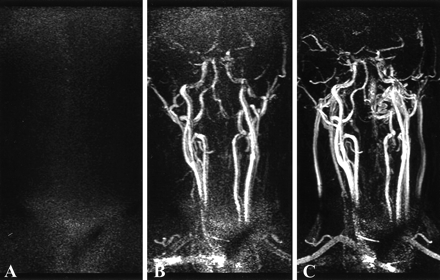
One important advantage of the SENSE technique over techniques that implement partial k-space sampling is that the former allows matching the duration of the intraarterial plateau concentration of contrast agent to the duration of one dynamic acquisition without compromise of image quality (image detail and SNR) or introduction of artifact. This is demonstrated in Figure 3, in which three consecutive images of the second volunteer showed the dynamic nature of the acquisition, including an arterial phase and an early mixed arteriovenous phase. On the other hand, in techniques where only partial k-space updating for each dynamic image is used to shorten the acquisition time, the duration of intraarterial plateau concentration of contrast agent cannot be limited to one dynamic acquisition, but should be maintained at least over the duration of multiple dynamic acquisitions used in view sharing and interpolation (5). This is done to prevent amplitude modulation in the shared k-space data resulting from variability in T1 and T2* shortening. Hence, at a fixed injection rate and similar dynamic acquisition time, the SENSE technique requires less contrast agent (less cost) to maintain shorter intraarterial plateau duration.
26-year-old healthy volunteer.
A–C, MIPs of three consecutive 3D contrast-enhanced MR angiography (TR/TE/flip angle: 4.9/2.4/35°) dynamic acquisitions demonstrate three different phases of the contrast bolus flowing through the neck vessels: mask (A), arterial phase (B), and mixed arterial and venous phase (C).
One limitation of the SENSE technique is related to the incomplete penetrability and coverage of the surface coils used, which resulted in poor imaging of the origins of the great vessels off the aorta. Better surface coil design with greater coverage and penetrability is needed for imaging of the great vessels.
Another method, known as simultaneous acquisition of spatial harmonics (SMASH), which implements a similar imaging technique to reduce acquisition time, has been used in body contrast-enhanced MR angiography (8). The basic difference between SENSE and SMASH is the reconstruction part. In SENSE, the unfolding of aliased pixels is done after Fourier transformation of the k-space data, while the simultaneous acquisition of spatial harmonics reconstruction is based on fitting the harmonic functions encoding k-space, prior to Fourier transformation. The latter will then give a general fitted solution to the problem of aliasing in the partially phase-encoded images, and therefore be global on the entire image, whereas SENSE is a pixel-by-pixel method, and therefore is possibly more computationally intense (8). On average, both methods use the sensitivity profiles of each receiver coil as a means to speed up image acquisition. In both cases, complex algorithms allow for optimization of SNR while minimizing aliasing artifacts (6, 8), resulting in similar image quality.
Conclusion
SENSE provides time-resolved, contrast-enhanced 3D carotid MR angiography with submillimeter in-plane resolution without the need for bolus timing or postacquisition segmentation.
Footnotes
1 Address reprint request to Elias R. Melhem, MD, Department of Radiology and Radiological Sciences, The Johns Hopkins Medical Institutions, 600 North Wolfe Street, Baltimore, MD 21287-2182.
References
- Received December 27, 2000.
- Accepted after revision April 2, 2001.
- Copyright © American Society of Neuroradiology