Abstract
BACKGROUND AND PURPOSE: The occurrence of damage in the entorhinal, perirhinal, and temporopolar cortices in unilateral drug-refractory temporal lobe epilepsy (TLE) was investigated with quantitative MR imaging.
METHODS: Volumes of the entorhinal, perirhinal, and temporopolar cortices were measured in 27 patients with unilateral drug-refractory TLE, 10 patients with extratemporal partial epilepsy, and 20 healthy control subjects. All patients with TLE were evaluated for epilepsy surgery and underwent operations.
RESULTS: In left TLE, the mean volume of the ipsilateral entorhinal cortex was reduced by 17% (P < .001 compared with control subjects) and that of the ipsilateral temporopolar cortex by 17% (P < .05). In right TLE, the mean ipsilateral entorhinal volume was reduced by 13% (P ≤ .01), but only in patients with hippocampal atrophy. Asymmetry ratios also indicated ipsilateral cortical atrophy. When each patient was analyzed individually, the volume of the ipsilateral hippocampus was reduced (≥ 2 SD from the mean of controls) in 63% and that of the entorhinal cortex in 52% of patients with TLE. Furthermore, ipsilateral entorhinal (left: r = 0.625, P < .001; right: r = 0.524, P ≤ .01), perirhinal (left: r = 0.471, P < .05), and temporopolar (right: r = 0.556, P < .01) volumes correlated with ipsilateral hippocampal volumes. There was no association, however, with clinically or pathologically identified causes of epilepsy, duration of epilepsy, or age at onset of epilepsy. Mean cortical volumes were unaffected in extratemporal partial epilepsy.
CONCLUSION: Subpopulations of patients with unilateral TLE have ipsilateral damage in the entorhinal and temporopolar cortices. The damage is associated with hippocampal damage.
The human medial temporal lobe is composed of the hippocampus, the amygdala, and the surrounding cortex, including the entorhinal, perirhinal, and parahippocampal cortices (1). Structural and electrophysiologic abnormalities associated with human temporal lobe epilepsy (TLE) are currently best understood in the hippocampus. Accordingly, our knowledge regarding seizure generation and epileptogenesis is based primarily on studies of the epileptic hippocampus (2). Despite a growing body of evidence indicating that temporal lobe areas other than the hippocampus are also damaged in TLE (3), little attention has been directed to the contribution of entorhinal, perirhinal, or temporopolar cortical damage to the pathophysiology of TLE.
The entorhinal cortex in humans lies in the anterior parahippocampal gyrus and comprises Brodmann's area 28 (4). Functionally, it serves as an interface between the hippocampus and the surrounding unimodal and multimodal sensory cortices (5). Recent observations in both rats and humans with TLE demonstrate neuronal loss in layer III of the entorhinal cortex (6, 7), which is associated with synaptic reorganization (8). Consistent with histologic observations, recent studies using quantitative MR imaging suggest that at least the entorhinal cortex is damaged in patients with TLE (9, 10).
The human perirhinal cortex is located along the collateral sulcus in the ventromedial aspect of the temporal lobe. It borders the entorhinal cortex laterally and comprises Brodmann's areas 35 and 36 (5). The rostromedial continuation of area 36 of the perirhinal cortex (area 36p) forms the temporopolar cortex as defined by Insausti and colleagues (5). The perirhinal cortex is the major input area to the entorhinal cortex and has heavy connections with the unimodal and polymodal cortical association areas as well as with the amygdala (11, 12). Considering this vast interconnectivity and the participation of the entorhinal and perirhinal cortices in the medial temporal lobe memory system (13), the occurrence of damage within these structures could contribute, in part, to the clinical symptomatology of TLE. Earlier volumetric MR imaging studies have reported either total volumes of the anterior temporal lobe (14–16) or changes in the total volumes of the temporal gray and white matter in patients with TLE (17, 18). Otherwise, quantitative MR imaging data regarding anatomically and functionally distinct areas of the perirhinal or temporopolar cortices in epilepsy are limited (19, 20).
The purpose of this study was to evaluate (a) whether the entorhinal, perirhinal, or temporopolar cortices are damaged in patients with chronic drug-refractory TLE by using a histology-based quantitative MR imaging method (21); (b) whether medial temporal cortical damage occurs in patients with extratemporal partial epilepsy; and (c) the factors associated with medial temporal cortical damage.
Methods
Patients and Control Subjects
The control group included 20 healthy individuals (10 women, 10 men), with a mean age of 32 ± 10 years (SD; range, 21–52 years).
There were 27 patients (12 female, 15 male patients) in the TLE group. They belonged to a consecutive series of patients who had undergone evaluation for epilepsy surgery and subsequently underwent operations at the Kuopio University Hospital between 1993 and 1997 for unilateral drug-refractory TLE. The presurgical evaluation included a neurologic evaluation, MR imaging, ictal video-electroencephalographic (EEG) recording (27 recordings with scalp and sphenoidal electrodes, eight recordings with subdural strip electrodes), neuropsychological evaluation, sodium amobarbital (Wada) test for the assessment of speech lateralization and memory, and psychiatric evaluation (n = 22). On the basis of video-EEG recordings, 12 patients had TLE with unilateral seizure focus on the left and 15 patients had seizure focus on the right. Postoperative outcome was assessed 1 year after the operation according to a classification adapted from Engel (22). Only patients with good postoperative outcome (Engel's classes I and II) were included.
At the time of the imaging, the mean age of the patients in the TLE group was 34 ± 10 years (range, 16–47 years) and the mean duration of epilepsy was 21 ± 14 years (range, 2–43 years). The mean age at the onset of epilepsy was 13 ± 12 years (range, 1–43 years). Preoperative seizure frequency varied from 17 to 672 seizures per year (median, 73) during the year preceding the operation. In a majority of patients (n = 25), most of the seizures were complex partial without secondary generalization (range, 15–672; median, 72). On the basis of preoperative clinical assessment, the cause of epilepsy was unknown (cryptogenic) in 15 patients. In 12 patients, the cause of epilepsy was considered symptomatic. The symptomatic etiologies included central nervous system infection (viral meningitis, viral encephalitis, measles or congenital toxoplasmosis; n = 4), asphyxia (n = 3), focal cortical dysplasia (n = 2), head trauma (n = 1), and ganglioglioma of the amygdala (n = 1). Additionally, one patient had dual pathology combining a history of surgery for cavernoma and hippocampal damage.
Ten patients (five female, five male patients) with well-localized intractable extratemporal partial epilepsy were included in the “extratemporal group.” Five patients had seizure focus in the frontal lobe; three, in the parietal lobe; and two, in the occipital lobe. The extratemporal seizure onset and the seizure symptomatology were verified with ictal video-EEG recordings (three recordings with scalp electrodes, three with subdural strip/grid electrodes) in six patients. Eventually, four patients underwent surgery with palliative indications, and postoperative outcome was assessed 1 year later. One patient achieved a worthwhile seizure reduction with complete resolution of complex partial and secondarily generalized seizures (only partial elementary auditory seizures continuing, Engel's class IIIA). Three patients did not benefit from the surgery (Engel's class IVA and B). Poor outcome in these patients was associated with the cause of epilepsy and extent of extratemporal resection. In the remaining four patients, the localization of seizure focus was based either on the seizure symptomatology, MR imaging, and postoperative outcome (Engel's class IA, n = 1) or on seizure symptomatology, MR or positron emission tomography imaging, and interictal EEG findings (n = 3).
The mean age at the time of imaging in the extratemporal group was 32 ± 12 years (range, 15–46 years). The mean age at onset of epilepsy was 14 ± 15 years (range, 1–40 years), and the mean duration of epilepsy was 19 ± 16 years (range, 3–44 years). The cause of epilepsy was cryptogenic in two and symptomatic in eight patients. Symptomatic causes included focal cortical dysplasia (n = 2), encephalitis (n = 2), asphyxia (n = 1), anoxia during epiglottitis (n = 1), hamartoma (n = 1), and cavernous hemangioma (n = 1). The seizure frequency at the time of MR imaging varied from 24 to 2190 seizures per year (median, 90).
This study was approved by the joint ethics committee for human research at the University of Kuopio and the Kuopio University Hospital.
MR Image Acquisition and Volumetric Analyses
Patients and control subjects underwent imaging with a 1.5-T Magnetom (Siemens, Erlangen, Germany) using a standard head coil and a tilted coronal 3D magnetization-prepared rapid acquisition gradient-echo sequence with the following parameters: 10/4/1 (TR/TE/excitation); inversion time, 250 ms; flip angle, 12 degrees; field of view, 250 mm; matrix, 256 × 192. This resulted in 128 contiguous T1-weighted images with a 1.5- to 2.0-mm section thickness oriented perpendicular to the long axis of the hippocampus.
The volumes of the entorhinal, perirhinal, and temporopolar cortices in all patients and control subjects were measured by the same investigator (L.J.) using a histology-based volumetric method (21). To minimize the effect of a possible bias, the cases were analyzed in random order without exact knowledge of the focus. The images throughout the entire rostrocaudal extent of the temporopolar, entorhinal, and perirhinal cortices were reconstructed into 2-mm-thick contiguous sections oriented perpendicular to the line drawn between the anterior and posterior commissures at the midsagittal level. Then, the following landmarks were identified from the images: the temporal pole, appearance and depth of the collateral sulcus, the limen insulae, and the last sections containing the entorhinal or perirhinal cortices. Thereafter, boundaries of the temporopolar, entorhinal, and perirhinal cortices were determined (for location of each area, see Fig 1). To reduce the error in tracing the boundaries, images were magnified and interpolated fourfold, which resulted in an effective pixel size of 0.25 mm. Finally, the outlines of each area were traced with a trackball-driven cursor on successive MR images from the rostral to caudal ends. The volumes were calculated with software developed in-house for a standard work console. In two patients with right TLE, one patient with left TLE, and one patient with extratemporal partial epilepsy, the boundaries of the right perirhinal cortex could not be reliably defined because of focal cortical dysplasia (n = 1) or movement artifacts (n = 3). The hippocampal volumes were measured (K.P.) as previously described (23). The intraobserver variability for hippocampal volumes was 6.8%.
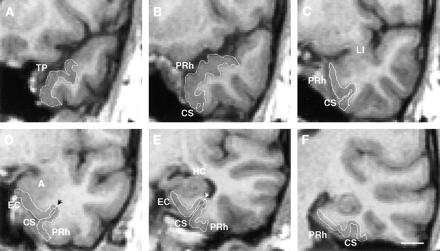
Coronal MR images from a control subject demonstrate the boundaries of the temporopolar, entorhinal, and perirhinal cortices (white outlines). Six rostrocaudal levels are shown (A, most rostral; F, most caudal).
A, The temporopolar cortex is the area between the lateral edge of the superior temporal sulcus and the medial bank of the inferior temporal sulcus.
B, The appearance of the collateral sulcus marks the beginning of the perirhinal cortex.
C, The boundaries of the perirhinal cortex at the level of the limen insula. The collateral sulcus is shallow (depth, < 1 cm), and therefore, the lateral border of the perirhinal cortex is located at the midpoint of the occipitotemporal gyrus (16).
D, The boundaries of the entorhinal and perirhinal cortices at the level of the amygdala. The collateral sulcus is shallow, and therefore, the border between the entorhinal and perirhinal cortices is located at the fundus of the collateral sulcus (arrowhead). The lateral border of the perirhinal cortex is located at the midpoint of the occipitotemporal gyrus.
E, The boundaries of the entorhinal and perirhinal cortices at the level of the hippocampus. The borders of the entorhinal and perirhinal cortices are identical to those in D.
F, The caudal limit of the perirhinal cortex is located two sections behind the end of the uncus. A indicates amygdala; CS, collateral sulcus; EC, entorhinal cortex; HC, hippocampus; LI, limen insula; PRh, perirhinal cortex; TP, temporopolar cortex. Scale bar, 10 mm.
Statistics
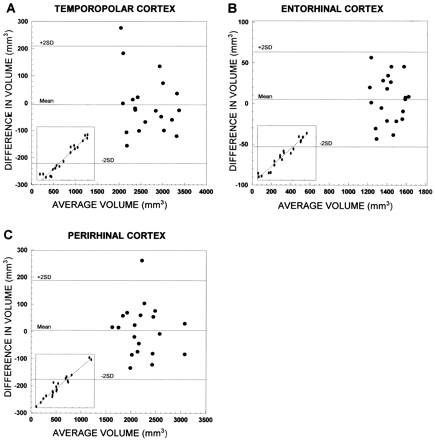
The data were analyzed with SPSS 9.0 software for Windows (SPSS Inc, Chicago, IL). To correct volumetric data for individual variance in head size, the raw volumes were normalized according to Cendes et al (16) with modifications (24). In brief, the mean brain area (obtained from the coronal image at the level of the anterior commissure; correlation with the brain volume: r = 0.67, P < .001, n = 20) of the control subjects was divided by the corresponding brain area of the patient. Each measured cortical volume was then multiplied by this ratio for each patient. The intraobserver variability of repeated measurements is described with the method introduced by Bland and Altman (25). Repeated measurements were performed for 10 control subjects. The limits of agreement between the first and second measurements were defined as the mean difference in volume (first-second measurement) ± 2 SD of this mean difference (Fig 2). The clinical significance of the intraobserver variability was assessed by comparing the limits of agreement with (a) the total volume of each measured area, (b) the mean volume reductions, and (c) the volume considered to be a marked volume reduction in individual analyses (≥ 2 SD from the mean of controls). The mean difference in volume was near zero in each measured area, and the limits of agreement were not considered clinically significant.
Scatterplots show the intraobserver variability of repeated measurements in different cortical areas of 10 control subjects. A, Temporopolar cortex; B, entorhinal cortex; and C, perirhinal cortex. The limits of agreement between the first and the second measurements are expressed as the mean difference in volume: [volume in the first measurement minus volume in the second measurement (mm3)] ± 2 SD. Inserts in the lower left corner show the association between the first (y-axis) and second (x-axis) measurements. Mean indicates mean difference in volume; +2 SD, mean difference in volume plus 2 SD; -2 SD, mean difference in volume minus 2 SD
For statistical analyses, the patients were divided into three groups according to localization of the seizure focus: TLE patients with the focus on the left, TLE patients with the focus on the right, and patients with extratemporal partial epilepsy. The initial statistical survey indicated that the variances of most volumetric parameters were unequal in different subgroups, and it was not possible to perform the analysis of variance between the groups. All parameters were normally distributed, however, as verified by using the Kolmogorov-Smirnov test for normal distribution. Therefore, the mean volumes of the entorhinal, perirhinal, and temporopolar cortices in different patient groups were compared with those in the control group by using the independent samples t test with Bonferroni adjustment (3×). Because the temporopolar cortex is the rostromedial continuation of the perirhinal cortex, the combined volumes of the perirhinal and temporopolar cortices (referred to as the total perirhinal cortex) were added to similar analyses. To assess the degree of asymmetry in the volumes, an asymmetry ratio was calculated according to Bernasconi and colleagues (9):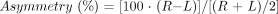 where R refers to the volume on the right and L that on the left. Asymmetry ratios in different patient groups were compared with those of the control group, and between left and right TLE groups, using the independent samples t test with Bonferroni adjustment (4×). The presence of cortical damage ipsilateral and contralateral to the side of seizure focus in individual patients was analyzed by using the volume considered to be a marked volume reduction (≥ 2 SD from the mean of controls) as a cutoff point.
where R refers to the volume on the right and L that on the left. Asymmetry ratios in different patient groups were compared with those of the control group, and between left and right TLE groups, using the independent samples t test with Bonferroni adjustment (4×). The presence of cortical damage ipsilateral and contralateral to the side of seizure focus in individual patients was analyzed by using the volume considered to be a marked volume reduction (≥ 2 SD from the mean of controls) as a cutoff point.
After the initial analyses, both TLE groups were divided into two subgroups according to the degree of hippocampal damage: patients with a reduction of at least 2 SD from the mean of control subjects in the ipsilateral hippocampal volume, and patients with a reduction of less than 2 SD in the ipsilateral hippocampal volume. Each subgroup was compared with the control group, and, additionally, subgroups of patients with left TLE and subgroups of patients with right TLE were compared with each other (within the TLE group). The comparisons were made using nonparametric Kruskal-Wallis and Mann-Whitney tests with Bonferroni adjustment (6×).
In control subjects, right-left asymmetry in the volumes was analyzed by paired samples t test, and the effect of sex was assessed with independent samples t test. The effect of age at the time of MR imaging, age at onset of epilepsy, and duration of epilepsy between groups were assessed with a one-way analysis of variance with Tukey's post hoc test. Differences in seizure frequency between groups were evaluated using nonparametric Kruskal-Wallis and Mann-Whitney tests with Bonferroni adjustment. The contribution of cause and complex febrile seizures to the damage was evaluated with the nonparametric Mann-Whitney test. The correlations were calculated with the two-tailed Pearson's correlation test. A P value of less than .05 was considered statistically significant.
Results
Control Subjects
The mean volumes of the entorhinal, perirhinal, temporopolar, and total perirhinal cortices in control subjects are shown in Table 1. There was no significant right-left asymmetry in the mean volumes. Additionally, the volumes were not affected by sex or age.
Normalized volumes of the left and right entorhinal, perirhinal, temporopolar, and total perirhinal cortices in control subjects and patient groups
Patients with Left TLE
Surgical outcome 1 year after the operation was excellent in nine patients, because four became completely seizure-free (Engel's class IA), four had only auras after surgery (Engel's class IB), and one only experienced a seizure during inappropriate drug withdrawal (Engel's class ID, seizure-free after medication was resumed). One patient had a good outcome with rare seizures (Engel's class IIA, fewer than three seizures per year). Additionally, two patients initially had a worthwhile seizure reduction (Engel's class IIIA, greater than 80% seizure reduction) after surgery. After a 3-year follow-up, one of these patients became seizure-free (Engel's class IC, some seizures after surgery but seizure-free for at least 2 years) and the other had good outcome (Engel's class IIC, more than rare seizure after surgery but rare seizures for at least 2 years). Thus, these patients also were included in the study.
The mean age and sex in the left TLE group did not differ from those in the control or other patient groups. Additionally, the mean age at onset of epilepsy, the mean duration of epilepsy, and the preoperative seizure frequency did not differ from those in the other epilepsy groups (see below). The mean hippocampal volume was reduced by 32% ipsilaterally (P < .001) but not contralaterally. The asymmetry ratio of the hippocampus was higher than that in control subjects (P ≤ .01) or in patients with right TLE (P < .001) (Table 2).
Asymmetry ratios of various medial temporal lobe areas in control subjects and patient groups
Volumes of Entorhinal, Perirhinal, and Temporopolar Cortices
The mean volumes of the entorhinal, perirhinal, temporopolar, and total perirhinal cortices are shown in Table 1. The presence of cortical damage ipsilateral and contralateral to the side of seizure focus in individual patients is summarized in Figure 3. Ipsilaterally, the mean volumes of the left entorhinal and temporopolar cortices were both reduced by 17% compared with control subjects (P < .001 and P < .05, respectively), whereas the mean volumes of the perirhinal and total perirhinal cortices did not differ from those in control subjects. All contralateral cortical mean volumes also were normal (Table 1). The asymmetry ratios of the entorhinal, perirhinal, and total perirhinal cortices were higher in the left TLE group than in the control group (P < .05, in all) or in the right TLE group (P < .001 in all), indicating smaller volumes ipsilateral to the seizure focus (Table 2).
Percentage of TLE patients with damage to the hippocampus (A), entorhinal cortex (B), temporopolar cortex (C), or perirhinal cortex (D). Contra indicates the side contralateral to the seizure focus; Dam+, a volume reduction of at least 2 SD from the control mean; Dam-, a volume reduction of less than 2 SD from the control mean; EC, entorhinal cortex; Focus, side of the seizure focus; HC, hippocampus; n, number of patients; PRh, perirhinal cortex; TP, temporopolar cortex
Patients with Right TLE
Surgical outcome 1 year after the operation was excellent in all patients with right TLE (Engel's class IA, completely seizure-free after surgery). The mean age, the mean age at onset of epilepsy, the mean duration of epilepsy, the sex distribution, and the preoperative seizure frequency did not differ from those in the control or other patient groups. The volume of the hippocampus was reduced by 31% ipsilaterally (P < .01) but not contralaterally when compared with control subjects. The asymmetry ratio of the hippocampus was lower in the right TLE group than in the control group (P ≤ .01) or the left TLE group (see above; Table 2).
Volumes of Entorhinal, Perirhinal, and Temporopolar Cortices
There were no differences in the volumes of the ipsilateral entorhinal, perirhinal, temporopolar, or total perirhinal cortices in the right TLE group when compared with those in control subjects. Also, all contralateral cortical mean volumes were unaffected (Table 1). The asymmetry ratios of the entorhinal, perirhinal, and total perirhinal cortices, however, were lower in the right TLE group than in control subjects (P < .01, P < .001, and P < .01, respectively) or the left TLE group (see above), indicating smaller volumes ipsilateral to the side of seizure focus (Table 2).
Patients with Extratemporal Partial Epilepsy
The mean volumes of the entorhinal, perirhinal, temporopolar, or total perirhinal cortices, as well as the mean hippocampal volumes in patients with extratemporal partial epilepsy did not differ from those in control subjects (Table 1). The corresponding asymmetry ratios also did not differ from those of the control group (Table 2).
Patients with Hippocampal Atrophy
Entorhinal, Perirhinal, and Temporopolar Volumes
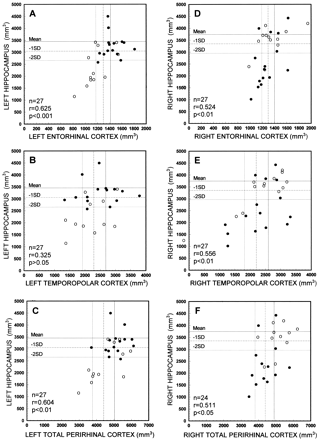
Next, we assessed whether the volume reduction within the entorhinal, perirhinal, or temporopolar cortices is associated with hippocampal atrophy. There was no correlation between the hippocampal volume and the volume of the ipsilateral/contralateral entorhinal, perirhinal, and temporopolar cortices in control subjects or patients with extratemporal partial epilepsy. In all patients with TLE, the volume of the left hippocampus correlated with the volume of left entorhinal (r = 0.625, P < .001), perirhinal (r = 0.471, P < .05), and total perirhinal cortices (r = 0.604, P < .01). The volume of the right hippocampus correlated with the volume of the right entorhinal (r = 0.524, P ≤ .01), temporopolar (r = 0.556, P < .01), and total perirhinal cortices (r = 0.511, P < .05) (Fig 4). The left or right hippocampal volumes did not correlate with the contralateral cortical volumes (data not shown).
Scatterplots show the correlation between the volumes of the left (A–C) or right (D–F) hippocampus and the volumes of the entorhinal, temporopolar, and total perirhinal (ie, combined perirhinal and temporopolar volumes) cortices on that side in patients with TLE. Closed circles refer to patients with right TLE and open circles to patients with left TLE. Mean indicates mean volume in control subjects; -1SD, mean volume in control subjects minus 1 SD; -2 SD, mean volume in control subjects minus 2 SD; n, number of patients; r, Pearson's correlation coefficient
To assess whether TLE patients with a hippocampal volume reduction of at least 2 SD on the side of the seizure focus have more substantial cortical damage than patients with milder hippocampal atrophy, the cortical volumes in the TLE subgroups were evaluated. Seven patients with left TLE had a volume reduction of at least 2 SD from the mean of control subjects (at least a 23% volume reduction) in the ipsilateral hippocampus, and the mean hippocampal volume was reduced by 49% on average compared with the control group (P < .001). Ipsilaterally, the mean volume of the left entorhinal cortex was reduced by 23% (P < .001) and the total perirhinal cortex by 22% (P < .05) compared with control subjects. The ipsilateral mean volume of left temporopolar and perirhinal cortices as well as all contralateral cortical mean volumes were unaffected (Table 3). The ipsilateral or contralateral cortical mean volumes in patients with milder hippocampal volume reduction did not differ from those in control subjects or from those in patients with more substantial hippocampal damage.
Normalized volumes of the left and right entorhinal, perirhinal, temporopolar, and total perirhinal cortices in control subjects and TLE patients with a reduction of at least 2 SD from the mean of control subjects in the ipsilateral hippocampal volume
Ten patients in the right TLE group had a volume reduction of at least 2 SD from the mean of control subjects (at least a 21% volume reduction) in the ipsilateral hippocampus (mean volume reduction of 48% compared with control subjects, P < .001). Ipsilaterally, the mean volume of the entorhinal cortex was reduced by 13% (P ≤ .01) compared with the control group. The ipsilateral mean volumes of the right perirhinal, temporopolar, and total perirhinal cortices, as well as all contralateral cortical mean volumes, did not differ from those in control subjects (Table 3 and Fig 5). The ipsilateral or contralateral mean volumes in patients with milder hippocampal volume reduction (< 2 SD from control means) did not differ from those in control subjects. Additionally, when the right TLE patients with mild or more severe hippocampal damage were compared with each other, there were no differences in the cortical mean volumes.
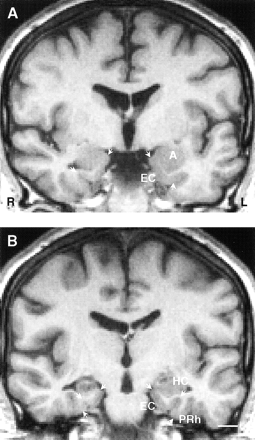
Coronal MR images of a 32-year-old man with cryptogenic right TLE. The duration of epilepsy at the time of imaging was 29 years, and the seizure frequency was 36 complex partial seizures per year. The patient subsequently underwent surgery and is currently seizure-free (Engel's class IA).
A, Ipsilaterally, the volume of the entorhinal cortex is 81% of that (mean volume) in control subjects and 78% of that on the contralateral side.
B, MR image taken from a more caudal level shows the volume reduction in the ipsilateral hippocampus (volume 41% of that in control subjects and 50% of that on the contralateral side). The volume of right perirhinal cortex is 2597 mm3; left, 2924 mm3 (both within normal range). A indicates amygdala; EC, entorhinal cortex; HC, hippocampus; L, left; PRh, perirhinal cortex; R, right. Scale bar, 10 mm.
Assessment of Other Candidate Factors Contributing to Damage
There were no differences in the ipsilateral or contralateral cortical volumes between patients with clinically determined symptomatic or cryptogenic TLE. The pathologic examination of the resected tissue revealed hippocampal damage in 21 patients (hippocampal sclerosis, n = 18; gliosis in the hippocampus, n = 2; hippocampal dysgenesis, n = 1). In the remaining six patients, the pathology of the hippocampus was either normal (n = 3) or the hippocampal tissue was not available for analysis (n = 3). Two larger pathologic entities were identified among TLE patients: patients with hippocampal sclerosis (or gliosis) and normal temporal cortex (n = 8), and patients with hippocampal sclerosis (or gliosis) and cortical microdysgenesis in the temporal cortex (n = 12). There were no differences in the ipsilateral or contralateral cortical volumes between these two pathologic entities. When the TLE patients with (n = 5) or without (n = 22) complex febrile seizures were compared using the nonparametric Mann-Whitney test, there was no difference in cortical volumes on the side of the focus. Finally, there was no correlation between the duration of epilepsy or age at onset of epilepsy and the cortical volumes.
Discussion
The purpose of this study was to investigate the occurrence of damage in the medial temporal cortex in patients with intractable TLE. The volumes of the entorhinal, perirhinal, and temporopolar cortices were quantified in a consecutive series of patients who were evaluated for epilepsy surgery and subsequently underwent operations because of unilateral drug-refractory TLE. Additionally, the volumes were measured in 10 patients with chronic extratemporal partial epilepsy. The cortical volumes were compared with those in healthy control subjects. The results indicate that subpopulations of patients with unilateral TLE have a reduction in the volumes of entorhinal and temporopolar cortices ipsilateral to the seizure focus. Second, the evaluation of asymmetry ratios suggests significant cortical asymmetry ipsilateral to the focus. Third, cortical damage is associated with hippocampal damage but not with clinically or pathologically identified causes of epilepsy, duration of epilepsy, or age at onset of epilepsy. Finally, the volumes of the medial temporal cortical structures were normal in patients with extratemporal partial epilepsy.
Entorhinal Volumetry
The volume of the ipsilateral entorhinal cortex was reduced in 52% of the patients with intractable TLE. In left TLE, the mean volume of the ipsilateral entorhinal cortex was reduced by 17%, whereas in right TLE there was a reduction in the mean entorhinal volume only in a subgroup of patients with marked hippocampal atrophy. In this patient population, entorhinal volumes clearly correlated with ipsilateral hippocampal volumes. Consistent with this observation, TLE patients with more severe (≥ 2 SD) hippocampal damage had the smallest mean entorhinal volumes ipsilaterally, and TLE patients with mild (< 2 SD) hippocampal damage had normal mean entorhinal volumes both ipsilaterally and contralaterally. The association of entorhinal damage with hippocampal damage was further supported by the finding that isolated ipsilateral entorhinal damage without hippocampal damage occurred in only three patients (11%). The number of patients in some subgroup analyses was small. The consistency of results from different analyses, however, strengthens the value of these observations.
Four previous MR imaging studies have quantified the volumes of the entorhinal cortex in patients with epilepsy (9, 10, 20, 26). The early attempts used a subtraction method for volume analyses (26). These authors reported that the mean volume of the rostral 40% of the parahippocampal gyrus (corresponding to the caudal half of the entorhinal cortex) was reduced ipsilateral to the focus, and that there was also an abnormal right-left difference in patients with chronic drug-refractory partial epilepsy. Using more accurate methodology, Bernasconi et al (9) reported a bilateral reduction in the mean volumes of the entorhinal cortex in patients with intractable TLE undergoing evaluation for surgery. Our recent observations in another patient population demonstrated a reduction in the volume of the entorhinal cortex in individual patients with cryptogenic TLE, particularly if the hippocampus also was damaged (10). The mean entorhinal volumes, however, were not affected ipsilaterally or contralaterally. The present study confirms and extends the previous observations by showing that a subpopulation of patients with drug-refractory TLE have a unilateral volume reduction in the entorhinal cortex, which in most (11 [79%] of 14) cases is associated with hippocampal damage. In the present study, entorhinal damage was bilateral in only two patients. Therefore, we could not confirm previous observations suggesting that the volume reduction in the entorhinal cortex in patients with unilateral drug-refractory TLE is bilateral (9). This difference from the results of Bernasconi et al might relate to a different patient population in terms of cause and degree of hippocampal damage. First, the cause of epilepsy in the study of Bernasconi and colleagues was not specified. Second, the mean hippocampal volume reduction in their study was more prominent than in the present study. Furthermore, in 37% of our patients, there was only mild or no hippocampal damage observed in the MR imaging and there was bilateral damage in only three patients (11%). In this respect, the present data are more equivalent to those of the study of Salmenperä et al (10), which included cryptogenic patients with or without hippocampal damage. Considering that the hippocampus and the entorhinal cortex are heavily interconnected via excitatory glutamatergic pathways (27), the data indicate that codamage of these two areas might relate to the seizure spread from one area to another (28). That the hippocampus was damaged in a higher percentage of patients (63%) than the entorhinal cortex (52%) might be the result of the higher sensitivity of the hippocampus to excitotoxic damage. Also, entorhinal volumetry might be less sensitive than hippocampal volumetry for detection of minor structural damage.
Perirhinal and Temporopolar Volumetry
The median perirhinal volumes and perirhinal volumes in individual patients were unaffected both in cases of TLE and extratemporal partial epilepsy. The mean volume of the ipsilateral temporopolar cortex was reduced by 17% in left TLE. In individual analyses, the volume of the temporopolar cortex was reduced ipsilateral to the seizure focus in 22% of patients with TLE. Unlike the entorhinal cortex, only the right temporopolar and left perirhinal volumes correlated with the ipsilateral hippocampal volumes.
Quantitative MR imaging data regarding anatomically and functionally distinct areas of the perirhinal or temporopolar cortices in human TLE are sparse (19, 20), with most studies reporting volumes of the entire anterior temporal lobe (14–18). Some studies indicate an ipsilateral reduction in the mean volume of the anterior temporal lobe (including the temporopolar cortex) in patients with TLE (14–16), and even suggest a correlation between the volume of the left temporal lobe and verbal memory performance (15). Other studies investigating the structural changes of the temporal lobe in patients with TLE suggest a bilateral reduction in the volume of the temporal neocortical gray matter (17, 18) as well as unilateral (18) or bilateral (17) reduction in the volume of the temporal white matter. Bernasconi and colleagues (19) reported a ≥ 2 SD perirhinal volume reduction in 33% of patients with unilateral intractable TLE, but the study included only six patients. Our data indicate that a substantial proportion of patients with refractory TLE have damage in some components of the polymodal sensory association cortices of the medial temporal lobe (52% in the entorhinal cortex, 22% in the temporopolar cortex). The contribution of the entorhinal and temporopolar damage to clinical symptomatology of TLE remains to be determined.
Factors Associated with Damage to the Medial Temporal Cortex
Seventeen patients with TLE (63%) had damage in some component of the medial temporal cortex, whereas only two patients with extratemporal epilepsy had a volume reduction of at least 2 SD in the entorhinal, perirhinal, or temporopolar cortices. Therefore, the location of the seizure focus in the temporal lobe was critical. The age at seizure onset was not associated with damage to the medial temporal cortex. Nor was there a correlation between the duration of epilepsy and cortical volumes. Some studies, however, indicate that the volume of the temporal gray matter (18) or entorhinal volume (10) correlates negatively with the duration of epilepsy.
We could not associate the medial temporal cortical damage in patients with TLE with any clinically or pathologically identified etiologic subgroup. Only five patients (19%) in the TLE group had a history of complex febrile seizures. Analysis of the mean cortical volumes indicated no differences in the side of the focus between patients with and those without a history of complex febrile seizures. In individual analyses, however, ipsilateral hippocampal damage was associated with cortical damage in three of these patients. Because previous studies have included patients with either cryptogenic (10) or unspecified (9) causes, further analyses of the different etiologic factors in a larger patient population are needed. A plausible hypothesis might be that patients with pathologically identified hippocampal sclerosis and abnormal temporal cortical pathology (eg, cortical microdysgenesis) have smaller cortical volumes than patients with hippocampal sclerosis and normal temporal cortical structures.
Medial Temporal Lobe Volumetry and Localization of Seizure Focus
When analyzed individually, there was at least a 2 SD volume reduction from the mean of control subjects in the ipsilateral hippocampus in 62% of our patients with TLE and in the ipsilateral entorhinal cortex in 52% of patients with TLE. On the other hand, there was a marked (≥ 2 SD) volume reduction in the contralateral hippocampus in 11%, and in the contralateral entorhinal cortex only in 7%, of patients with TLE. Considering that cortical damage was associated with hippocampal damage in TLE, the sensitivity of hippocampal volumetry will probably remain superior to the entorhinal volumetry in assessing the location of the focus in candidates for epilepsy surgery. Quantitative volumetry of the entorhinal cortex, however, could provide additional independent information about the seizure focus in selected candidates for epilepsy surgery. First, isolated entorhinal damage ipsilateral to the seizure focus was found in 11% of patients with TLE. Second, unilateral entorhinal volume reduction was not observed in patients with extratemporal partial epilepsy. Therefore, entorhinal volumetry could be useful, for example, in patients in which there is no clear hippocampal or lesional damage observed on MR imaging. Also, entorhinal volumetry might help in the differentiation between TLE and extratemporal epilepsy in problematic cases.
The volumetry of the temporopolar and perirhinal cortices was clearly less sensitive in lateralizing the seizure focus in TLE. There was ipsilateral damage in the temporopolar cortex in only a small subgroup (22%) of patients with TLE. There also was quite a substantial variation in volumes of the temporopolar and perirhinal cortices already among healthy individuals, which is consistent with previous observations (21). Furthermore, the method for assessing the volumes of the perirhinal and temporopolar cortices is technically more demanding than for hippocampal volumetry.
For now, the association between hippocampal volumetry and postoperative surgical outcome in TLE is well established (29). Additional information about the possible predictive value of the entorhinal volumetry in assessing the outcome of epilepsy surgery is needed. Whether the damage in the entorhinal and temporopolar cortices contributes to the memory impairment in TLE remains to be explored as well.
Conclusion
Our data indicate that subpopulations of patients with unilateral TLE have ipsilateral damage in the entorhinal and temporopolar cortices. The damage is associated with hippocampal damage, but not with cause, duration of epilepsy, or age at onset of epilepsy. In patients with TLE, there was a volume reduction of at least 2 SD from the mean of control subjects in the ipsilateral hippocampus in 63% of patients; in the entorhinal cortex, 52% of patients; and in the temporopolar cortex, 22% of patients. The possible predictive value of entorhinal volumetry in assessing the outcome of epilepsy surgery, however, is unclear. From a functional point of view, further studies also are needed to reveal how entorhinal and temporopolar damage relates to the EEG pattern of seizure generation and spread, and to clinical symptomatology, including memory impairment.
Acknowledgments
We thank Dr. Tuuli Salmenperä for her help in conducting MR volumetry and her constructive criticism of the final version of the manuscript. The superb statistical expertise of Pirjo Halonen, MSc, is greatly acknowledged.
Footnotes
1 Address reprint requests to Dr. Asla Pitkänen, A. I. Virtanen Institute, University of Kuopio, P.O. Box 1627, FIN-70211 Kuopio, Finland.
This study was supported by the Academy of Finalnd, the Vaajasalo Foundation, the Sigrid Juselius Foundation, the Kuopio University Hospital, and the University of Kuopio.
References
- Received January 19, 2001.
- Accepted after revision April 16, 2001.
- Copyright © American Society of Neuroradiology