Abstract
Summary: Presentation, diagnosis, and management of an unusual parasagittal ependymoma, radiographically resembling a falcine meningioma, are described. Despite its radiographic appearance, pathologic evaluation revealed classic features of an ependymoma. The radiographic and pathologic characteristics of this unusual lesion are briefly examined, and the literature is reviewed. Although extraaxial ependymomas are rare, they should be considered in the radiographic differential diagnosis of dural-based lesions, especially for patients within the first 3 decades of life.
Ependymomas represent 2%–9% of all intracranial tumors. These tumors typically present in the pediatric population (mean age range, 8–25 years) (1). Approximately two thirds of these lesions arise in the midline posterior fossa, whereas one third of them can be found supratentorially. Although most supratentorial ependymomas are believed to arise from the ventricular system, a significant minority of these lesions are primarily intraaxial in nature without clear-cut ventricular involvement. The origin of such intraaxial ependymomas is controversial. Certain authors postulate that these intraparenchymal lesions develop from extensions of the ventricular surface that have subsequently regressed (2). The more conventional opinion is that intraaxial, extraventricular ependymomas originate from ependymal embryologic remnants (3, 4).
Primary extraaxial ependymomas, however, very rarely occur. To our knowledge, only four such cases have been described in the literature (2, 3, 5, 6). We present the unique case of an extraaxial, dural-based ependymoma without parenchymal involvement that was radiographically identical to a parasagittal, falcine meningioma.
Case Report
A 20-year-old man presented with a history of secondarily generalizing, partial seizures involving the right upper extremity since age 9. His initial workup did not reveal an anatomic etiology, and his seizures were originally treated successfully with phenobarbital and carbamazepine. During the 2 months before presentation, the patient's seizure frequency increased and he experienced progressively worsening headaches. He also had difficulties with fine motor coordination of the right upper extremity and with speech during his postictal periods.
The patient appeared healthy upon neurologic examination. Cranial nerve, motor, and sensory examination did not show focal deficit, with the exception of mild hyperreflexia of the right upper and lower extremities compared with the left side. The patient's toes were down-going to plantar stimulation, and there were no other long-tract signs.
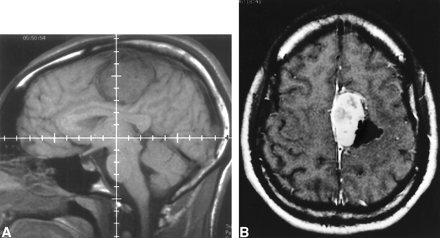
MR imaging revealed an extraaxial, 5 × 3 × 3.2-cm, left- sided, parafalcine mass adjacent to the precentral gyrus. The bulk of the lesion was isointense to brain on T1-weighted images, isointense to brain on T2-weighted images with a hyperintense rim, and densely contrast-enhancing in a heterogeneous fashion following gadolinium administration (Fig 1). The lateral portion of the mass demonstrated low T1 and T2 signal consistent with dense mineralization (Figs 1–3). An MR venogram demonstrated a patent superior sagittal sinus with absence of abnormal flow voids. The preoperative radiographic diagnosis favored falcine meningioma.
A, Sagittal T1-weighted precontrast MR imaging at 580/18 (TR/TE) and B, axial T1-weighted postcontrast MR imaging at 720/20 (TR/TE) reveal parafalcine mass with heterogeneous contrast enhancement and dense calcification laterally. Asterisk in B indicates contrast-enhancing dural tail
Operation
A left-sided, parietal craniotomy was performed via a horseshoe-shaped incision crossing the midline. Intraoperative sonography was used to identify the mass, and the dura was opened in a curvilinear fashion directly over the lesion. The medial precentral gyrus was gently retracted laterally, and the densely calcified portion of the tumor was removed piecemeal using rongeurs. The lesion was noted to originate from the falx without frank parenchymal invasion. With the cutting Bovie loop (Cadman; Johnson & Johnson, Raynham, MA), the mass was removed in a gross total fashion, and the portion of the falx from which the tumor arose was coagulated using bipolar cautery. Postoperatively the patient had marked right lower extremity weakness that significantly improved with inpatient rehabilitation. One month after discharge, the patient returned with only a trace of weakness during right- foot dorsiflexion. Total-spine MR imaging did not show evidence of central nervous system dissemination. Follow-up brain MR imaging 1 year postoperatively did not show evidence of residual or recurrent tumor.
Pathologic Examination
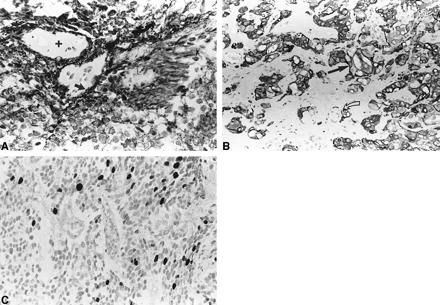
Characteristic regions of this heterogeneous ependymoma showed perivascular rosettes that were positive for glial fibrillary acidic protein (GFAP) (Fig 4A). Other regions showed clear cells and vacuolated cells. Despite a superficial resemblance to chordoma or chordoid meningioma, these vacuolated cells were positive for GFAP (Fig 4B). Neoplastic cells were negative for cytokeratin, neurofilaments, and synaptophysin. Rare isolated cells showed epithelial membrane antigen. Focal mitoses, vascular proliferation, necrosis, and Molecular Immunology Borstel (MIB-1) proliferation indices ranging from 0% to 18% in different microscopic fields (Fig 4C) suggested an aggressive potential. These features contrasted with its well-differentiated histology and cytologic appearance.
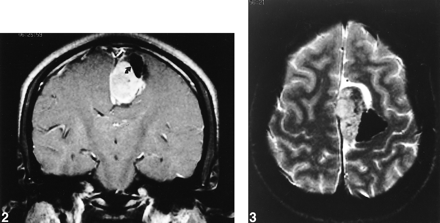
Coronal, T1-weighted, postcontrast MR imaging at 850/22 (TR/TE) further delineates parasagittal mass. Curved arrow points to region of dense calcification.fig 3. Axial T2-weighted MR imaging at 2500/80 (TR/TE) reveals dense mineralization in lateral aspect of mass. High cerebrospinal fluid signal surrounding mass emphasizes extraparenchymal nature of lesion
A, GFAP highlights intensely positive glial fibrils in perivascular rosettes. Other cells have clear cytoplasm. Nuclei are round and oval with finely speckled chromatin.
Plus sign is in vessel lumen; solid arrow points to perivascular GFAP positivity.
B, Groups of vacuolated cells are positive for GFAP (open curved arrow) and spread between a hyalinized matrix (solid arrow).
C, MIB-1 proliferation index is focally high, up to 18%.
Discussion
Intracerebral ependymomas represent 5% of all intracranial tumors in adults (4). Approximately one third of these intracranial ependymomas are supratentorial. Age is a key factor in their development as supratentorial ependymomas occur more frequently in the adult population (7). Approximately 50% of supratentorial ependymomas originate in the ventricular system. The remaining ependymomas occur in the parenchyma without obvious association with the ventricular system (4). The prevailing theory holds that these ependymomas originate from fetal rests of ependymal cells.
Extraaxial location of supratentorial ependymomas is extremely rare. In our review of the literature, we found only four reported cases of extraaxial ependymomas. Two of these cases were extraaxial, infratentorial tumors (3, 6). The remaining two cases involved extraaxial, supratentorial ependymomas. Hanchey et al (5) reported the case of a 29-year-old patient with a large interhemispheric ependymoma, imaged with cerebral angiography and Technetium 99m scintigraphy. The authors report that, although extraaxial, this tumor appeared to extend toward the lateral ventricles. This case was reported in the era before detailed axial imaging was possible. It is, therefore, more difficult to assess the relationship of this lesion to the ventricular surface. Hayashi et al (2) reported the case of a 13-year-old patient with a left occipital ependymoma that was predominately extraaxial. No obvious connection to the ventricular system was found, although the tumor did not appear to be completely extraaxial. Unlike either of these two cases, our unique case involves a completely extraaxial, supratentorial, dural-based ependymoma. No apparent parenchymal extension was noted intraoperatively, and the parenchyma appeared normal in character.
No definitive mechanism for the development of these extraaxial ependymomas has been postulated. One hypothesis involves the extension of subcortical, subependymal rests extraaxially with the subsequent growth of tumor (2). Necrosis and calcification of the originating subependymal rests would then follow, leaving a predominately extraaxial ependymoma. Donich et al (6) reported the case of an extraaxial cerebellopontine angle ependymoma extending to the cavernous sinus without obvious connection to the ventricular system. These authors theorized that a microscopic cellular tract likely existed between the ventricular system and the extraaxial ependymoma. Heterotopic placement of ependymal cell rests during fetal development with subsequent growth of tumor was also discussed as a possible mechanism. Given the location and the lack of parenchymal involvement, we favor the heterotopic placement of ependymal cell rests in the falx as the origin of the tumor in our case.
Radiographically, extraaxial ependymomas can be difficult to differentiate from other dural-based lesions. Even classic lesions in the posterior fossa can be variable in appearance. As with meningiomas, ependymomas can present with isointense signal on T1- and T2-weighted images. However, contrast enhancement of ependymomas, unlike meningiomas, is usually inhomogeneous. The lesions are generally well circumscribed with cystic regions and various degrees of calcification. Ependymomas are sometimes difficult to distinguish from other gliomas on the basis of signal characteristics alone. Consequently, the location of the lesion is of significant importance in distinguishing the differential diagnosis.
The management of intracranial ependymomas nearly always begins with surgery, with the goal being complete resection (1, 6, 8). The need for postoperative radiation therapy is varied, and the topic is controversial. In general, postoperative radiotherapy is thought to have a significant impact on increasing the length of survival, especially for patients with subtotal resections. Little distinction regarding whether the location of the tumor is a factor in the need for postoperative radiation has been made. In a small study, Palma et al (8) concluded that radical surgery alone was satisfactory for selected supratentorial ependymomas. These ependymomas were completely resected and were not of high grade. In our case, a complete resection was accomplished and, therefore, no postoperative radiation was given. After 16 months of follow-up, there is no apparent recurrence of tumor.
Various prognostic factors have been presented in the literature, including age, tumor location, histology, and the extent of resection. Most studies show increased survival with gross total resection. Various studies have reported increased survival with presentation at an older age for infratentorial ependymomas (9). However, in the case of supratentorial ependymomas, there is evidence that older age at presentation is not necessarily associated with prolonged survival (7). The literature is inconsistent about whether tumor location (supratentorial vs infratentorial) significantly impacts survival, although there have been reports of decreased survival with a supratentorial location. In a recent retrospective study of supratentorial ependymomas, Schwartz et al concluded that the most significant negative predictors for survival were association with the third ventricle and metastatic disease (7).
Extraaxial ependymomas are so rare that they may be overlooked during pathologic differential diagnosis. They often exhibit unusual structural features. For these reasons, immunohistochemical evaluation is very useful in their evaluation. Glial fibrillary acidic protein is a critical marker. Testing positive for GFAP eliminates major alternative possibilities, including meningioma, and highlights glial processes in perivascular rosettes. Occasional schwannomas express GFAP but not perivascular rosettes. Other gliomas express GFAP, and oligodendrogliomas have round nuclei in scant and clear cytoplasm that resemble ependymoma. However, oligodendrogliomas lack epithelial membrane antigen and perivascular rosettes. Central neurocytomas have structures like ependymomas but express neuronal markers and lack GFAP.
Conclusion
The etiology of extraaxial, supratentorial ependymomas is unclear. This particular case gives credence to the theory that these lesions derive from ependymal cell rests and not from extensions of the ependymal surface that have subsequentially regressed. Immunohistochemistry plays a crucial role in establishing the diagnosis and in differentiating these lesions from more common dural-based lesions. While extraaxial, supratentorial, ependymomas are extremely rare, they should be considered in the differential diagnosis of dural-based lesions, especially for patients within the first 3 decades of life.
Footnotes
1 Address reprint requests to Andrew S. Youkilis, MD, The University of Michigan, Section of Neurosurgery, 1500 E. Medical Center Drive, 2128 TC, Box 0338, Ann Arbor, MI 48109.
- Received August 17, 2000.
- Copyright © American Society of Neuroradiology