Abstract
BACKGROUND AND PURPOSE: Follow-up imaging data from stroke patients without angiographically apparent arterial occlusions at symptom onset are lacking. We reviewed our Emergency Management of Stroke (EMS) trial experience to determine the clinical and imaging outcomes of patients with ischemic stroke who showed no arterial occlusion on angiograms obtained within 4 hours of symptom onset.
METHODS: All patients in this report were participants in the EMS trial that was designed to address the safety and potential efficacy of combined IV and intraarterial thrombolytic therapy with recombinant tissue plasminogen activator (rt-PA) in patients with acute ischemic stroke.
RESULTS: Thirty-five patients were randomized to receive either IV rt-PA (n = 17) or placebo (n = 18), followed by cerebral angiography. No symptomatic arterial occlusion was evident in 10 (29%) of the 34 patients. Eight (80%) of 10 patients without angiographically apparent clot within 4 hours of symptom onset had a new cerebral infarction confirmed on follow-up brain imaging. The median 72-hour infarction volume was 2.4 cc (range, 1–30 cc). Four of the 10 “no-clot” patients had a favorable 3-month outcome as assessed by Barthel Index (score, 95 or 100) and modified Rankin Scale (score, 0 or 1). The six remaining patients had 3-month Rankin Scale scores of 1 (Barthel of 90), 2, 3, 4, or 5.
CONCLUSION: Acute ischemic stroke patients with a neurologic deficit but a negative angiogram during the first 4 hours after symptom onset usually develop image-documented cerebral infarction, and approximately half suffer from long-term functional disability. The two most likely explanations for negative angiograms are very early irreversible ischemic damage despite recanalization or ongoing ischemia secondary to clot in non-visible penetrating arterioles or in the microvasculature.
Several investigators have recommended that stroke patients with a clinical deficit who do not have angiographically documented arterial occlusion should not be treated with a thrombolytic agent (1–4). The underlying assumption for this recommendation is that early lysis of the occluding thrombus has already occurred in these patients, and use of thrombolytic therapy in this setting increases the risk of intracerebral hemorrhage without any potential additional benefit. However, occlusions of small cerebral arteries are difficult, if not impossible, to visualize angiographically, and can lead to cerebral infarction and long-term neurologic disability. In the present study, we report on the imaging and long-term clinical outcomes of patients who participated in the Emergency Management of Stroke (EMS) bridging trial (5) and who did not manifest arterial occlusion on angiograms. This trial was conducted prior to the Food and Drug Administration approval of recombinant tissue plasminogen activator (rt-PA) for the treatment of acute ischemic strokes within 3 hours of onset.
Methods
Subjects
This pilot, double-blind, randomized, placebo-controlled, multicenter phase I study was designed to address the safety and potential efficacy of combined IV and intraarterial thrombolytic therapy with rt-PA in patients with acute ischemic stroke within 3 hours of symptom onset (5). Informed consent was obtained from each patient, his/her legal guardian, or his/her next of kin. Institutional review boards approved the protocol at each institution.
Inclusion and Exclusion Criteria
The study inclusion and exclusion criteria of the EMS study have been published previously (5).
Randomization and Treatment
Patients were randomized within 3 hours of symptom onset to receive IV rt-PA (Activase alteplase: Genentech Corp., South San Francisco, CA) at 0.6 mg/kg (60-mg maximum, 10% of dose as bolus over 1 minute) or placebo over 30 minutes, followed immediately by cerebral angiography. If a clot was identified that was appropriate for the patient's symptoms, intraarterial rt-PA (two 1-mg boluses, followed by infusion of 10 mg/h,20-mg maximum) was administered via catheter. If clot was not identified, intraarterial rt-PA was not given, even if the patient's neurologic deficit persisted. The protocol required that no anticoagulants or antiplatelet agents be given during the first 24 hours after intraarterial therapy and that blood pressure be maintained within prespecified values as per National Institute of Neurologic Disease and Stroke (NINDS) guidelines (6).
Catheterization and Intraarterial Protocol
An introducing catheter was placed in the femoral artery by using a one-wall puncture after the IV study medication had been infused. If the suspected distribution of ischemia was the carotid artery, injection into the common carotid artery for carotid bifurcation and intracranial examination was performed. If the suspected arterial distribution was the vertebral or basilar artery, injection of the vertebral arteries was performed. Final arteriography included ipsilateral anteroposterior and lateral carotid injections or the vertebrobasilar system in anteroposterior and lateral projections if this was suspected to involve the arterial system (digital subtraction arteriography or cut-film arteriography). In patients with a suspected carotid distribution stroke, a contralateral carotid and a vertebral injection were not required. In patients with a vertebrobasilar distribution stroke, carotid injections were not required. Intravenous heparin was administered as a bolus (4000 U) at the beginning of the procedure. If at the time of the initial angiogram the patient did not show evidence of occlusion in the vascular territory appropriate for his/her symptoms, no intraarterial rt-PA was administered, and the procedure was terminated.
Monitoring Adverse Events
Safety was evaluated by monitoring all bleeding complications, particularly the incidence of CT-documented intracranial hemorrhage as well as large groin and retroperitoneal meatomas or gastrointestinal bleeding.
Follow-up and Measurement of Outcome
The National Institutes of Health Stroke Scale (NIHSS) was performed at baseline, at 72 hours ± 6 hours, 7 days ± 24 hours, and 90 days ± 7 days. Outcome measurements at 3 months ± 7 days included Glasgow Outcome Scale, Barthel Index, modified Rankin Scale, and NIHSS (5). Noncontrast brain CT scanning was performed at baseline at 72 hours ± 6 hours and at 7 days ± 24 hours. Emergent CT scanning of the brain was performed for any signs of acute neurologic deterioration. MR imaging of the brain and MR angiography of the intracranial vessels could be performed at the discretion of the treating physician, but was not part of the study protocol.
A careful search for potential cardiac sources of embolus was made in each patient, including transthoracic echocardiography. Each patient was categorized as to the most likely source of clot that resulted in the stroke (atherothrombotic, atheroembolic, cardioembolic, small-vessel occlusion, or other less common cause) after completion of all diagnostic studies. The criteria for small-vessel occlusion and cardioembolism were the same as those used in the NINDS t-PA stroke trial (6). The subtyping of the stroke was done by clinicians at each institution. In addition to the treating neuroradiologist at the clinical center, all cerebral angiograms and baseline 72-hour and 7-day CT scans were evaluated by the coordinating center neuroradiologist (T.A.T.) who was blinded to clinical findings and institution. In the initial EMS trial article (5), we reported on the initial impression of the treating neuroradiologist regarding the presence or absence of arterial clot, because this evaluation was used to determine whether intraarterial rt-PA should be given. Subsequently, the central neuroradiologist judged that two patients treated with IV rt-PA, who were originally judged by the treating neuroradiologist at the time of treatment to have no visible occlusion directly related to the ischemia requiring thrombolysis, did have arterial occlusion. One patient with an aortic dissection appeared to have “sludge,” or embolic material in distal arterial branches that resulted in very slow flow in the distal branches of the middle cerebral artery. The other patient had occlusion of the distal vertebral artery that the treating physician determined did not warrant thrombolysis. The central neuroradiologist's evaluation of the baseline angiogram is used for the present analysis. Measurement of CT infarct volume was performed by the method of A × B × C/2, as previously reported (7).
Results
Thirty-five patients were randomized to receive either IV rt-PA (n = 17) or IV placebo (n = 18), followed by immediate cerebral angiography. Intraarterial rt-PA was administered only if a clot was identified. One patient did not undergo arteriography owing to femoral artery inaccessibility. No arteriographic procedure was terminated because of clinical deterioration attributable to an angiographic mass effect or CT-revealed hemorrhage.
Baseline Characteristics
Baseline characteristics of the EMS bridging trial patients without arterial occlusion are presented in Table 1. The median time (25th percentile, 75th percentile) from symptom onset to IV placebo or IV rt-PA was 2 hours 42 minutes (1 hour 47 minutes, 2 hours 57 minutes). The median (25th percentile, 75th percentile) time from onset of symptoms to cerebral angiography was 3 hours 11 minutes (2 hours 25 minutes, 3 hours 30 minutes).
EMS patients without clot—baseline characteristics and 72-hour imaging data
Angiographic Data
No symptomatic intracerebral arterial occlusion was demonstrated in 10 (29%) of 34 patients (puncture of the femoral artery was not technically possible in one patient). Of the 10 “no-clot” patients, three (30%) had received IV rt-PA and seven (70%) had received IV placebo. Two patients suspected to have carotid distribution ischemia had normal carotid arteriographic findings. Subsequent spin-echo MR imaging revealed posterior circulation infarctions, although subsequent MR angiography did not show any vessel occlusion in the vertebrobasilar territory.
Brain Imaging Data
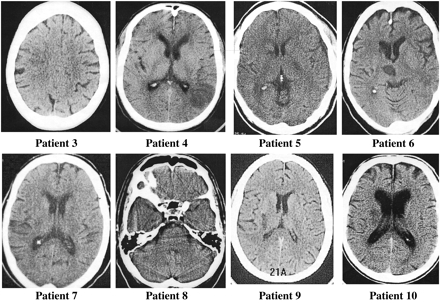
Brain imaging data are presented in Table 1. Eight (80%) of the 10 patients without angiographically evident arterial occlusion within 4 hours of symptom onset had new cerebral infarction revealed by follow-up brain imaging (brain CT or MR imaging) (Fig 1). The other two patients showed no evidence of cerebral infarction on follow-up CT scans, but did manifest inconclusive MR findings that depicted multiple zones of ischemic change of indeterminate age, some of which were in clinically appropriate vascular distributions. The median (25th percentile, 75th percentile) volume of cerebral infarction on the 72-hour CT scan was 2.4 cc (2.1, 5.8). The vascular distribution of definite cerebral infarction was in the internal carotid artery territory in six patients and in the vertebrobasilar territory in two patients.
Eight patients (patients 3–10 in Table 1) with CT-revealed infarcts at follow-up who did not manifest arterial occlusion on earlier angiograms.
No symptomatic hemorrhagic conversion was observed in the 10 “no-clot” patients. Asymptomatic hemorrhagic conversion was observed in two (20%) of the patients on CT scans at day 7 (one patient in the IV rt-PA group and one patient in the IV placebo group).
Suspected Etiology
The suspected etiologies of cerebral infarction in the 10 “no-clot” patients were small-vessel occlusion in three patients; cardiac embolism in two patients; (coronary catheter-induced) embolism in one patient; and unknown in four patients.
Safety Monitoring
No large groin or retroperitoneal hematoma was observed in the 10 “no-clot” patients. Groin oozing was noted in one patient in the IV rt-PA group.
NIHSS Assessment and Three-month Outcome
Baseline, 72-hour, and 7-day NIHSS score assessments are presented in Table 2. Three-month outcome, as assessed by the Barthel Index, the modified Rankin Scale, and the Glasgow Outcome Scale, is also presented in Table 2. The 3-month mortality rate in the 10 “no-clot” patients was 10% (one patient in the IV rt-PA group died at day 87 of metastatic breast cancer).
EMS patients without clot—3-month outcome
Discussion
The present study shows that clinically significant brain infarction commonly occurs in patients with normal angiograms within 4 hours of symptom onset. Eight of the 10 EMS trial patients without angiographically discernable clot showed a new cerebral infarction on follow-up brain images. CT revealed new infarction in seven of the patients and MR imaging did in one. In the two other patients without CT-revealed cerebral infarction, MR studies showed ischemic abnormalities of indeterminate age, potentially consistent with patients' symptoms. Diffusion-weighted MR imaging was not yet available.
The 72-hour infarction volume was variable, ranging from 1 cc in one patient to 30 cc in another patient who had a normal angiogram that was performed 83 minutes after symptom onset. The latter patient is likely to have had a major middle cerebral artery territory vessel occlusion and early recanalization. The median 72-hour infarction volume was small (2.4 cc) and seven of the “no-clot” patients developed cerebral infarctions of 7 cc or less. These smaller infarcts were located in deep areas supplied by small penetrating arterioles that are difficult, if not impossible, to visualize by cerebral angiography. Thus, there are two likely explanations for the findings of a normal cerebral angiogram within 3 to 4 hours of symptom onset and subsequent definite clinical and radiographic cerebral infarction at 72 hours: 1) very early irreversible ischemic damage despite early recanalization; or 2) ongoing ischemia secondary to clot in non-visible penetrating arterioles or in the microvasculature.
Although the absence of an angiographically confirmed arterial occlusion in our study was generally associated with small volumes of cerebral infarction, the associated disability was significant in five of the 10 “no-clot” patients. Only four of the EMS trial “no-clot” patients had a favorable3-month neurologic outcome as assessed by the Barthel Index (scores, 95 or 100) and a modified Rankin Scale (scores, 0 or 1). One other patient had a Rankin score of 1 but a Barthel score of 90. However, five of five patients with an NIHSS less than 10 had favorable outcomes. The patients in this study are an excellent illustration that prevention of even small infarcts, if they are in critical brain locations, will prevent substantial disability in some patients. Such patients typically have cerebral infarctions, often in the vascular distribution of poorly visualized, small, penetrating arteries, and they may suffer from long-term neurologic disability.
Arterial occlusion is present in at least 75% of ischemic stroke patients who undergo cerebral angiography within 6 to 8 hours of stroke onset (3, 4). Previous studies have note emphasized the analysis of imaging and clinical outcome of patients who did not have image-documented occlusion. The proportion of patients without angiographically evident occlusion (29%) observed in the EMS trial (median time from symptom onset to angiography, 191 minutes) was similar to the proportion of patients without arterial occlusion (19%) in the Prolyse in Acute Cerebral Thromboembolism II (PROACT II) trial (4). However, the design and timing of angiography in the two studies was different. As part of the design of the EMS trial, three patients without a demonstrable clot by angiography had received IV rt-PA prior to angiography and may have had early therapeutic recanalization prior to visualization of the cerebral arteries. No patients in PROACT received IV pro-urokinase prior to angiography. Second, in the EMS trial, the protocol of catheterization was guided by the clinical diagnosis of an anterior circulation or a posterior circulation stroke. Eight of the 10 EMS “no-clot” patients underwent cerebral angiography with an injection in the arterial territory consistent with the neurologic symptoms. A symptomatic thrombus may have been missed in two of the EMS “no-clot” patients, because a four-vessel angiogram was not required (the two patients with vertebrobasilar artery territory stroke did not have a vertebral injection). Follow-up MR angiography did not show any vertebrobasilar occlusion in these two patients. This emphasized the threat of ascribing posterior circulation occlusions to carotid distribution ischemia, pointing to the imperfect predictive value of the clinical examination. In contrast, PROACT focused only on patients with suspected focal ischemia in the distribution of the middle cerebral artery. Finally, the median time from symptom onset to angiography in the EMS trial (191 minutes) was substantially shorter than was the PROACT median time.
Angiography is an integral part of intraarterial thrombolysis and remains the standard of reference for visualization of the occlusion site and collateral circulation extent in acute ischemic stroke. However, angiography does not quantify tissue ischemia or reperfusion, and additional insight into the extent of the parenchymal ischemic lesion would be helpful in the management of patients without an angiographically demonstrable occlusion. In some patients in whom combined angiographic and single-photon emission CT were performed (8), arterial patency after thrombolytic therapy in ischemic stroke was not associated with tissue reperfusion. This discordance between arterial patency and absence of cerebral reperfusion may occur when emboli have lodged in the microvasculature, which is not angiographically visible. Ultrafast diffusion- or perfusion-weighted MR imaging has the potential to detect occlusion if microvasculature reperfusion has occurred or if cerebral tissue remains at risk from ongoing ischemia within the time window for thrombolytic therapy (9–14). The clinical utility of imaging cerebral blood flow or perfusion (xenon CT, diffusion- and perfusion-weighted MR imaging) could be tested in acute ischemic stroke patients without angiographically confirmed occlusion in future trials. The use of these imaging techniques to identify major arterial occlusions (internal carotid artery, M1 segment, M2 segment, vertebral artery, or basilar artery) and exclude patients with patent, large vessels may be supported in patients with lower NIHSS.
In conclusion, this study demonstrates that acute stroke patients with a persistent modest neurologic deficit but a negative angiogram during the first 4 hours after symptom onset usually develop CT- or MR-revealed evidence of cerebral infarction on follow-up brain images and frequently have long-term functional disability. The two most likely explanations for “normal” angiograms in these stroke patients are 1) very early irreversible ischemic damage despite recanalization or 2) ongoing ischemia secondary to clot in non-visible penetrating arterioles or in the microvasculature.
Footnotes
↵1 Address reprint requests to Thomas A. Tomsick, M.D., Department of Radiology, Section of Neuroradiology, P.O. Box 670762, University of Cincinnati Medical Center, Cincinnati, OH 45267-0762.
References
- Received June 22, 2000.
- Copyright © American Society of Neuroradiology