Acrylic cements have been used for the augmentation of weakened or partially destroyed bones for decades (1). The term vertebroplasty originally described an open surgical procedure that introduces bone graft or acrylic cement to mechanically augment weakened vertebral bodies. Polymethylmethacrylate (PMMA) is the acrylic most commonly used as a bone filler, and its application in the treatment of pathologic vertebral compression fractures (VCFs) has been reported extensively (1–10). In particular, it has been used to treat VCFs created by metastatic disease and primary bone tumors, such as aggressive hemangiomas and giant cell tumors (11).
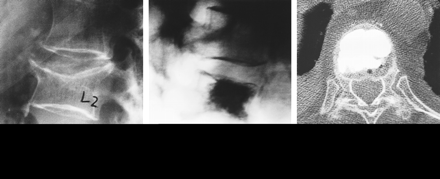
The first image-guided percutaneous vertebral augmentation, or percutaneous vertebroplasty (PVP), was performed in France in 1984, when Deramond and Galibert (12) injected PMMA into a C2 vertebra that had been partially destroyed by an aggressive hemangioma. The procedure relieved the patient's long-term pain. Shortly thereafter, PVP was used to treat VCFs caused by osteoporosis (13) (Fig 1).
Typical osteoporotic VCF at L2.
A, Preoperative radiograph, lateral view.
B, Radiograph after treatment with vertebroplasty. The dark area represents PMMA opacified with barium sulfate.
C, Axial CT scan of the treated L2 segment.
The interest in PVP has continued to grow since its introduction in Europe and its subsequent introduction in the United States by the interventional neuroradiology team at the University of Virginia (14). PVP reportedly offers the patient rapid relief from the pain associated with VCFs and is evolving as a standard of care for VCFs (15). In this review, we describe patient selection criteria, technical aspects of the procedure, and potential complications; review some of the basic science and biomechanics research related to the procedure; and present some future hurdles that still must be overcome for PVP to become a fully accepted standard of care.
Demographics of Vertebral Compression Fracture
VCFs occur when the combined axial and bending loads on the spine exceed the strength of the vertebral body (16). Reduction in the individual vertebral body strength may result from infiltrative processes created by benign or malignant tumors or, more commonly, from bone mineral loss precipitated by osteoporosis (17–19). Osteoporosis, which may be age-related (primary) or due to steroid use (secondary), is the most common cause of VCF in the United States (20).
VCF may be defined as either a radiographic or a symptomatic clinical event (21). The prevalence of radiographic VCF has been reported by Melton et al (22) to be as high as 26% in women 50 years old or older. The frequency of radiographic evidence of VCF increases from 500 per 100,000 person years (py) in women aged 50 to 54 years to 2960 per 100,000 py in women more than 85 years old. Radiographic changes may be present without the patient experiencing pain.
Cooper et al (23) found an age- and sex-adjusted incidence of clinically symptomatic VCF of 123 per 100,000 py for the years 1985 to 1989 in a population-based study in Rochester, MN (men and women of all ages combined). The age-adjusted incidence in women (153 per 100,000 py) was almost double that for men (81 per 100,000 py) (23). Even so, the high incidence in men contradicts the common misconception that osteoporosis is a women's health problem. The incidence of VCF exceeded the age- and sex-adjusted rate (114 per 100,000 py) for hip fractures (23).
Patients with VCFs may experience severe and prolonged pain that can markedly alter activities of daily living. In the United States, VCFs account for 150,000 hospital admissions, 161,000 physician office visits, and more than 5 million restricted activity days annually (20). In addition, it has now been shown that patients with VCF may experience other related comorbid events. For example, Schlaich et al (24) found a significantly lower vital capacity and forced expiratory volume in patients who had incurred spinal osteoporotic fractures as compared with control subjects without such fractures. Kado et al (25) reported higher mortality rates in women with VCFs as compared with age-matched control subjects without fracture. Women with one or more fractures had a 1.23-fold greater age-adjusted mortality rate, and mortality rose as the number of VCFs increased: women with five or more fractures had a more than twofold increase.
VCF may also result from tumor infiltration, but the rate of occurrence is hard to assess accurately. Osteolytic metastases and myeloma are the most frequent malignant lesions of the spine (9). Improved cancer treatments have prolonged the life span of patients with primary tumors but have also increased the number of patients who subsequently experience metastatic vertebral involvement and collapse. In addition, because treatment of malignant lesions often includes the use of glucocorticoids, secondary osteoporosis may develop and result in VCFs.
Patient Selection for Vertebroplasty
The primary indication of PVP is pain caused by a VCF resulting from either osteoporosis or tumor infiltration (14, 26–33). Currently, we lack the data to support the prophylactic use of PVP in vertebrae at risk of fracture. Although the high probability of VCF in these patients is well known, the precise vertebrae that may become fractured cannot be predicted with sufficient accuracy to justify prophylactic intervention (34). Prophylactic intervention may evolve as more physiological cements or agents capable of stimulating bone remodeling are developed and become available. Substantial pain may also be associated with a clinically aggressive vertebral hemangioma with or without VCF. Although this situation is uncommon relative to osteoporotic and malignant VCF-related pain, it, too, is effectively treated with PVP (33, 34).
The timing of intervention for VCF pain must be individualized. Initially, therapeutic intervention with PVP was limited to those patients for whom nonoperative therapy (eg, analgesics, bed rest, physical therapy, bracing) had failed (14, 15, 26, 27), and early intervention was reserved for those who had side effects (eg, pneumonia, thrombophlebitis, and intolerance to narcotic analgesics) resulting from their disability or who were nonambulatory because of pain refractory to analgesics (35). However, early intervention with PVP may be indicated because of the low complication rates associated with the procedure in patients with osteoporotic VCFs (14, 15, 31, 36) and because of the possibility of continued collapse of the fractured vertebra during the often lengthy period of nonoperative therapy (14, 15, 26–33).
Before initiating PVP, one should carefully assess the patient to ensure that the pain is related to a VCF. Other spinal entities, such as facet arthropathy, herniated nucleus pulposus, and spinal stenosis, may also be present and complicate the evaluation. VCF pain usually worsens with weightbearing and improves with recumbency. VCF pain is typically localized to the area of the fracture and lacks radicular qualities that suggest nerve root or cord compression. Local palpation over the posterior elements of the involved vertebral body will often elicit pain and, combined with imaging verification of the anatomic site of compression, will aid in localization.
The presence of multiple VCFs makes it difficult to ensure accurate localization via standard radiographs and physical examination alone. Pain localization by physical examination should not be assumed to be more accurate than one vertebra above or below the level of maximal discomfort. Also, differentiating acute from chronic VCF on standard radiographs is impossible without comparison films with which to date the fractures. Therefore, in almost all complex situations with multiple compressions or prolonged pain after an inciting event, MR imaging should be used. MR images show both anatomic vertebral collapse and loss of normal signal from the marrow space of vertebrae with acute fractures. These findings are well seen on sagittal T1-weighted sequences because the edema associated with the compression produces a low (dark) signal compared with the high (bright) signal normally seen in the marrow fat. Heavily T2-weighted sequences, such as sagittal short-tau inversion recovery sequences, are the most sensitive, with fluid representing marrow edema. Standard T2-weighted fast spin-echo sequences without fat saturation pulses are often insensitive to marrow edema because of the relatively high signal intensity from fatty marrow. One should be cautious about treating vertebrae that exhibit normal signal on MR images, because many of these patients experience little or no pain relief after PVP. Careful patient selection increases the likelihood of good treatment results.
Bone scans may also be useful to assess problematic VCF(s), but we prefer MR imaging whenever possible because the latter offers exquisite anatomic detail and concurrent information about other abnormalities, such as spinal stenosis, that impact decisions about the use of PVP. Bone scans are sensitive in detecting VCFs, and a negative bone scan, like a negative MR image, indicates a low likelihood of pain relief after PVP therapy. Bone scans, however, can be positive long after substantial healing of a VCF has occurred. This fact, coupled with the more restricted anatomic information it affords (as compared with MR imaging), makes bone scans our choice only when MR imaging cannot be performed.
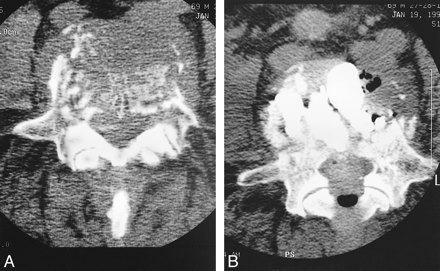
As is the case with VCFs, tumor invasion into the vertebral marrow space will also cause a loss of T1 signal on MR images. The presence of a tumor should be suspected when the entire vertebral marrow space appears dark on MR images. On the other hand, in the presence of osteoporosis, only the area immediately adjacent to collapse is dark. There is, however, overlap of signal alteration with this finding, making the low signal on T1-weighted images not totally specific. Similarly, radionuclide bone scans, although sensitive to both fracture and tumor invasion, are unable to differentiate a specific pathogenesis. Therefore, when a tumor is suspected, it may be appropriate to precede PVP with a biopsy. After the cannula is placed for PVP but before cement is injected, a coaxial biopsy specimen may easily be obtained. Pain caused by VCF due to tumor invasion is also effectively treated with PVP; therefore, PVP may immediately follow the biopsy (Fig 2). PVP does not hinder the therapeutic result of subsequent or concomitant radiation or chemotherapy.
A and B, Pre- (A) and postoperative (B) axial CT studies in a patient with an L5 vertebra destroyed by renal cell carcinoma who experienced pain relief 24 hours after PVP (which filled much of the vertebra with PMMA)
Vertebroplasty Technique
High-quality fluoroscopic equipment is essential for the safe performance of PVP. The cannula must be placed accurately to avoid collateral damage, and the cement must be observed during injection to prevent extravasation. Biplane fluoroscopic equipment allows the procedure to be performed more rapidly, but it can also be accomplished safely with a single-plane C-arm (34). CT has been described as an aid to fluoroscopy (36), but it adds considerable complexity and cost to the procedure without corresponding benefit to the routine treatment of a VCF. The use of CT is of benefit in the cervical spine (to avoid the carotid vessels during an anterolateral approach) and down to approximately T4 (where lateral fluoroscopy may be impossible through the shoulders). Regardless of the system used, it is essential to visualize the cement injection in real time (or after the injection of a fraction of a cubic centimeter of cement) to avoid excessive extravasation of cement, which presents the most likely potential for complications (29, 37, 38).
Patients typically receive intravenous antibiotics 30 minutes before the start of the procedure. Although this has become routine, its efficacy has never been validated in a controlled study. Prophylactic preoperative antibiotics (typically 1 g of cephazolin 30 minutes before the procedure) are routinely administered to patients undergoing open surgical procedures that entail PMMA implantation. Antibiotics may also be administered in the cement. Such delivery is usually used for patients who are known to be immunocompromised, but some clinicians routinely mix 1.2 g of tobramycin into the cement for all patients. This practice is empirical, and because it has not proved to be necessary (the occurrence rate of infection associated with PVP is very low), it cannot be scientifically or economically justified.
We typically use fentanyl (Sublimase, Abbott Labs, Chicago, IL) and midazolam (Versed, Roche, Manati, PR) to provide conscious sedation. Conscious sedation does not remove the need for adequate local anesthesia, which should affect not only the skin and subcutaneous tissues but also the periosteum of the vertebral body into which the cannula will be introduced. General anesthetics are seldom administered in patients in whom fluoroscopic guidance is used. With CT, however, patient movement is more critical, and general anesthesia is usually necessary. In addition, general anesthesia may be required for patients undergoing treatment of numerous levels, in which a lengthy operative time is necessary. (We believe that the treatment of more than three levels at one time is relatively contraindicated because of the potential risk of large quantities of marrow elements being embolized to the lungs.)
The area to be treated is prepared in a strictly sterile manner (as if placing a sterile port), with sterile drapes used over other regions. All personnel in the procedure room must observe a full sterile protocol. A posterior oblique projection that places the pedicle of the vertebra over the vertebral body is chosen, and local anesthetic is injected into the overlying skin, needle tract, and periosteum of the involved bone. A small dermatotomy is made with a scalpel. Using fluoroscopic guidance, an 11-gauge trocar and cannula system bone biopsy needle is introduced through the dermatotomy site to the posterior element of the vertebra. A small osteotomy is made in the bone with the cannula, which is then passed through the pedicle and into the vertebral body anterior to the midline of the body (as observed in the lateral projection). The position is checked in both anteroposterior and lateral projections during needle introduction. Alternative approaches include a) parapedicular cannula insertion in the thoracic spine between the rib head and lateral margin of the pedicle and b) posterolateral cannula insertion in the lumbar spine. Compared with the transpedicular approach, the parapedicular and posterolateral approaches may be more highly associated with cement leakage from the vertebral body when the trocar is removed. An anterolateral approach is needed in the cervical spine, and care must be taken to avoid the carotid and vertebral arteries during trocar insertion.
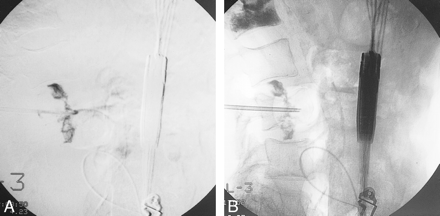
Venography is considered helpful and is routinely used during PVP by some physicians in the United States, but it is not commonly used in Europe. In the United States, it was adopted as a means of assessing cannula location for risk of cement leakage outside the vertebra; however, there are limitations to this method. First, contrast material and bone cement have different flow characteristics, so it is unknown whether there is accurate correlation between the flow path of the two agents. Second, even with the demonstration of extracorporeal venous filling, physicians rarely modify their techniques on the basis of information gained from venograms. High-resolution, real-time visualization of the cement and termination of the injection when a leak is observed provide maximum safety to the patient during the procedure. Third, some types of fractures, such as those that affect the endplates, can leak contrast material into the disk. During venography, these leaks may fill with contrast agent, which remains in place after the procedure, precluding early detection of PMMA if a similar leak occurs (Fig 3). Because of these considerations, some of us do not use venography.
Venographic studies of an osteoporotic VCF.
A, Lateral digital subtraction venogram shows nonionic radiographic contrast material leaking into both adjacent disks through endplate fractures.
B, Lateral venogram without digital subtraction of the same site, obtained approximately 10 minutes after A. Note that the radiographic contrast agent is still apparent and thus may have impeded visualization of possible cement leaks during PVP.
Currently, PVP is performed with some type of PMMA, such as Simplex P (Stryker-Howmedica-Osteonics, Rutherford, NJ), Osteobond (Zimmer, Warsaw, IN), or Cranioplastic (CMW, Blackpool, England). Only Simplex P is approved by the United States Food and Drug Administration (FDA) for use in pathologic fractures, including those in the spine. Simplex P and Osteobond contain 10% wt/vol of barium sulfate for opacification; however, this amount is insufficient for easy visualization during fluoroscopically guided PVP (33). Cranioplastic contains no barium sulfate and is not intrinsically radiopaque. Therefore, all PMMA cements that are currently available commercially require the addition of opacifier in sufficient quantity to ensure visualization and safe injection under fluoroscopy. In Europe, tungsten and tantalum powder are commonly used opacifiers (33), but these substances are difficult to obtain in sterile, medical grade in the United States and they are not approved by the FDA as opacifiers for PMMA cement. Therefore, in the United States, sterile barium sulfate has been the predominant choice as a cement opacifier. Approximately 30% wt/vol of barium sulfate must be added to PMMA powder to provide sufficient opacification for fluoroscopic monitoring (Jasper LE, Deramond H, Mathis JM, Belkoff SM, unpublished data, 2000). The barium sulfate must be pure, as defined by the United States Pharmacopoeia, and must not contain additives such as those commonly present in barium used for gastrointestinal evaluations. Barium sulfate requires sterilization by dry heat or radiation; ethylene oxide and steam are not acceptable methods of sterilization.
Biomechanical tests have shown that the addition of barium sulfate powder to the cement changes its strength; however, the strength of the cement with barium sulfate added to produce a 30% wt/vol mixture produces no practical change in the compressive strength of the PMMA (39), and it is doubtful that this change is clinically significant, because no mechanical failures of vertebrae treated by PVP have been reported.
Visualization of the cement during injection and careful monitoring for cement extravasation are the keys to making PVP safe. Even though small leaks (eg, a fraction of a cubic centimeter) may be tolerated without clinical sequelae, every attempt should be made to avoid extravasation. If biplane fluoroscopic equipment is used, visualization can easily be maintained in two projections during injection. With only single-plane equipment, the lateral projection must be monitored constantly, because this view allows rapid identification of cement leaks into the epidural space. One must still periodically check the anteroposterior projections to ensure that lateral leaks are avoided. If CT or MR imaging is used, real-time monitoring of the cement during injection is more difficult. Currently, most clinicians who use these imaging guidance methods for needle placement revert to fluoroscopic guidance during cement injection (36).
Once the procedure is completed, the patient should be maintained recumbent to prevent weightbearing while the cement hardens. PMMA cements typically set within 20 minutes and achieve approximately 90% of their ultimate strength within 1 hour of injection (40). Antibiotic ointment should be applied to the needle introduction sites and then covered with a sterile bandage, similar to skin care given to the injection site after angiography. If the procedure is performed on an outpatient basis, as is now common in the United States, the patient should be observed in the recovery area for 1 to 3 hours after surgery. Patients commonly experience pain relief between 4 and 24 hours postoperatively; however, local tenderness and minimal bruising at the puncture site are common and should be explained to the patient and family before and after the procedure. Typically, patients are medicated with analgesics before the procedure and are maintained on these medications after the procedure as needed. We routinely perform clinical follow-up examinations with repeat visual analog pain scale testing at 1, 7, and 30 days after PVP and compare these values with the results of preoperative testing. Additional radiographic evaluation is conducted only if the patient has not responded positively to the treatment.
Results and Complications
To date, no prospective, randomized trials comparing PVP with nonoperative medical therapy have been reported. Although the reports that do exist must be considered anecdotal, they are uniformly positive about the ability of PVP to produce pain relief for VCF with low complication rates. In the United States, Jensen et al (14) reported 90% pain relief in 29 patients treated with PVP for osteoporotic VCF. The only complications were two rib fractures. Notably, there were no neurologic complications. In a study of 38 patients, Barr et al (35) reported that 63% had marked pain relief and 32% had moderate pain relief after PVP. Only 5% of their patients experienced no improvement after PVP. (Note, however, that all the patients were treated with PVP only after nonoperative management had failed.)
Clinical complications reported after PVP include transitory fever, transient worsening of pain, radiculopathy, rib fractures, cement pulmonary embolism, infection, and spinal cord compression. The rate of complications varies considerably with the indications for PVP. With osteoporotic VCFs, complications are few (usually 1% to 2%) and most often are nonneurologic and transient (14, 15, 30, 35). In patients with malignant tumors, the complication rate increases owing to the risk of leakage of cement resulting from the vertebral body destruction caused by the primary malignant process. Transient radiculopathy has been reported in 3% to 6% of cases and has been successfully treated with steroids and antiinflammatory medications (27, 29, 32, 33); however, persistent radiculopathy occurred in 2% to 3% of patients and required surgical intervention for cement removal. Infection was reported only once, occurring in an immunocompromised patient (27).
Existing Hurdles
Several problems must be overcome before PVP can become the standard of care for the treatment of pain associated with VCF, most importantly the lack of an FDA-approved cement that contains adequate opacification for fluoroscopic monitoring. Several manufacturers are developing cement formulations that will accomplish this goal, but the regulatory process may delay the availability of an adequately opacified cement. As of this writing, several states have approved Medicare reimbursement for PVP, even with the complexities involving available cements. Such approval has largely been based on the observed efficacy and low complication rate of PVP, coupled with the general lack of an equivalent alternative.
Along with the need for optimal materials for PVP is the coexistent need for scientific validation through prospective randomized clinical trials that compare PVP with conventional medical therapy. Although the published studies are anecdotal and not randomized, they report rapid pain control in a high percentage of responders (12–14, 26–33, 36). In addition, these results have been achieved with a very low complication rate in the case of osteoporotic VCFs (14, 26, 27, 32, 33).
Studies that provide scientific validation may also provide better data about guidelines for appropriate use. The partially compressed, painful VCF seems an obvious choice. More information is needed, however, about the treatment of chronic fractures, fractures at multiple levels, extreme VCFs, and prophylactic use for at-risk vertebrae.
Biomechanics
Several bench-top studies have been conducted to gain a better understanding of the underlying mechanics responsible for the success of PVP as well as basic information on the materials used in the procedure. Such investigations are giving impetus to the development of new devices and materials to improve efficacy of the treatment (41, 42).
Several mechanisms have been proposed to explain the pain relief associated with vertebroplasty, including thermal necrosis and chemotoxicity of the intraosseous pain receptors as well as mechanical stabilization (43). It has been hypothesized that the monomer leachate may be neurotoxic (43–46). This is of particular concern with PVP because, to create a less viscous cement with a longer working time, more monomer is typically added to the powder than is recommended by the manufacturer (14, 37, 47). Such toxicity may also account for the necrotic zone reported around an injected tumor (48). The necrotic zone may also be caused by thermal damage resulting from the exothermic polymerization reaction of PMMA cement (48). During polymerization, temperatures can reach 122°C for large quantities of cement (49). A recent in vitro study of osteoporotic cadaveric vertebrae (50) suggested, however, that temperatures generated during vertebroplasty are not likely to be sufficient to result in widespread thermal necrosis of osteoblasts (51, 52) or neural tissue (53). There is also little risk of thermal damage to the spinal cord or nerve roots, provided the cement is contained within the vertebral body (50). Because tumor tissue may be more sensitive to the thermal effects of the cement than normal tissue is, temperature may still play a role in PVP for the treatment of tumors. Another possible mechanism responsible for the zone of necrosis in tumors is ischemia, which may result from direct (cement intrusion) or indirect (compression) occlusion of tumor vessels.
The most likely mechanism for pain relief after PVP treatment of osteoporotic VCFs appears to be mechanical stabilization of the vertebral body (39, 41, 50, 54). In most ex vivo studies, injection of cement into the vertebral body nearly restored its stiffness and increased its strength (39, 41, 54). We hypothesize that the injected cement prevents painful micromotion at the fracture site.
Currently, no cement has been approved by the FDA specifically for use in vertebroplasty. Of the cements commercially available for human use, all require alteration of their composition to make them suitable for vertebroplasty. To decrease viscosity and increase cement injectability, more monomer has been added to the cement powder than is recommended by the manufacturer (14, 37, 47). Changing the monomer-to-powder ratio can reduce the elastic modulus, yield strength, and ultimate strength of PMMA by as much as 24% (47); however this alteration in cement material properties is apparently of no clinical importance, as we have found no reports of complications attributed to cement failure or to insufficient cement strength or stiffness.
The various cements used in vertebroplasty possess different material properties, both in bench-top tests (Jasper LE, Deramond H, Mathis JM, Belkoff SM, unpublished data, 2000) and when injected into cadaveric specimens (39, 41, 54). Of the commercially available cements, Simplex P has exhibited the strongest and stiffest repair of in vitro specimens (39). Cranioplastic, a commonly used cement for vertebroplasty in the United States, exhibits the lowest strength and stiffness, yet still results in good clinical outcomes (14, 26). In addition, Cotten et al (29) found no correlation between the volume of cement injected and clinical outcome. We have learned from clinical practice that less than a complete vertebral body fill usually provides good pain relief (14, 26, 29). As with other orthopedic devices used to aid fracture healing, the goal of PVP is to provide stability to the vertebral body during the process of fracture healing. In this sense, PVP should be viewed more as a fracture repair technique than as an implant. Recently, research has indicated that a relatively small amount of PMMA is needed to restore prefracture strength to cadaveric vertebrae: 4.4, 3.1, and 2.5 mL into the lumbar, thoracolumbar, and thoracic regions, respectively (55).
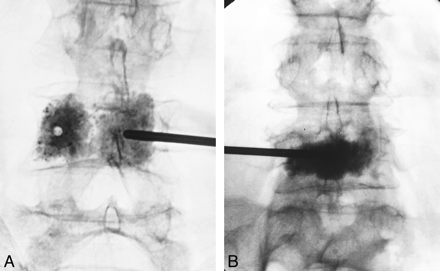
Originally, PVP was performed via a bipedicular injection of PMMA (Fig 4A). The question subsequently arose as to whether a unipedicular injection of cement alone could provide sufficient stabilization. A recent bench-top study performed to evaluate this possibility found that unipedicular injections (Fig 4B) that achieve fill across the midline can provide substantial restoration of strength (54). In a recent clinical study, half of the patients were treated with PVP by unipedicular injection, and there were no complications associated with this method of injection (35); however, small volume fills that do not distribute cement across the central vertical axis of the vertebral body should not be considered biomechanically adequate.
Injection techniques.
A, Anterolateral radiograph shows vertebroplasty performed via a bipedicular approach and injections.B, Anterolateral radiograph shows vertebroplasty performed via a single parapedicular approach, which resulted in a central needle position and good filling with one injection of cement
Future Directions
Within the next several years, new, non-PMMA bone cements will be available that will allow the testing of PVP as a treatment for new indications, which may include more aggressive therapy for neoplastic disease of bone by providing concurrent strength augmentation and chemotherapeutic potential. Percutaneous augmentation will most likely develop for areas other than the spine; for example, some preliminary data indicate that percutaneous cement augmentation may provide pain relief for metastatic involvement of the pelvis.
At this time, the primary goal of PVP is pain relief. Height restoration with reduction of kyphosis, along with pain control, is likely to play a larger role in the future. A percutaneously introduced inflatable bone tamp has been developed (Kyphon Inc, Santa Clara, CA) and has FDA approval. Although the device has been tested in vitro (42, 56), as yet there are no published findings in humans to indicate its efficacy or to delineate its indications or contraindications for use as compared with standard PVP. Indeed, height restoration has been reported to occur spontaneously (57) and has been achieved by one of us during PVP by using simple distraction before cement introduction while the patient was on the procedure table. The seemingly elementary technique of distraction may provide adequate height restoration without the need for additional devices or complexity.
Investigation of the prophylactic treatment of vertebrae at risk is just beginning. Because of the increased risk of additional fractures in patients with osteoporosis after their first VCF (25, 58), there may be a substantial need for prophylactic augmentation to prevent future collapse in these at-risk vertebrae. Epidemiologic and bone densitometric data may help provide the information needed to select the patients and the specific vertebrae that would most benefit from augmentation. Hydroxyapatite cements (that potentially can be remodeled into bone), osteoconductive cements, or bone stimulants (such as bone morphogenic protein) may find applications in this area. New materials may be introduced into the bone by using smaller cannulas than those now in common use. In addition, robotic or stereotactic guidance may improve the speed and accuracy of cannula placement.
Conclusions
To date, PVP has provided pain relief related to VCF with a very low complication rate. We believe that it will continue to gain scientific and patient acceptance and that it may ultimately become the standard of care for the treatment of painful VCFs. The use of percutaneous injection of cement to mechanically augment the skeleton may expand considerably beyond its currently limited application in the spine after VCF.
Footnotes
↵1 Address reprint requests to Stephen M. Belkoff, PhD, c/o Elaine P. Bulson, Editor, Department of Orthopaedic Surgery, Johns Hopkins Bayview Medical Center, 4940 Eastern Ave, #030, Baltimore, MD 21224-2780.
References
- Received July 6, 2000.
- Copyright © American Society of Neuroradiology