Abstract
BACKGROUND AND PURPOSE: The most frequent and serious complications of endovascular treatment of intracranial aneurysms with Guglielmi detachable coils (GDCs) are ischemic lesions caused by thromboembolic events. Diffusion-weighted MR imaging appears to be the most sensitive technique for detecting early ischemic phenomena. We evaluated this technique for the detection of brain changes in patients who underwent GDC treatment of aneurysms.
METHODS: Twenty patients with a cerebral aneurysm were studied with diffusion-weighted imaging before and after endovascular treatment with GDCs. Aneurysms were located in the anterior (n = 16) or posterior (n = 4) circulation. Bleeding had occurred in 11 patients. MR studies, including fast fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted sequences, were scheduled before, 2 to 4 hours after, and 48 hours after treatment. MR images, including apparent diffusion coefficient (ADC) maps, were assessed for the presence of acute ischemic stroke lesions.
RESULTS: In all patients, the aneurysm was excluded without neurologic worsening. In 18 patients, diffusion-weighted and FLAIR images showed no evidence of recent ischemic lesions after treatment. In one patient, an asymptomatic frontobasal hyperintense signal on diffusion-weighted images with a drop of ADC values corresponding to an acute ischemic lesion was observed. In another patient, multiple silent lesions were seen on diffusion-weighted images after embolization. These silent lesions were not all located in the vascular territory of the aneurysm's parent artery.
CONCLUSION: This preliminary study suggests that diffusion-weighted MR imaging is a potentially useful tool for monitoring patients after endovascular treatment of a cerebral aneurysm. While small asymptomatic lesions can be observed on these images after embolization, their exact prevalence should be evaluated in a larger series.
Endovascular techniques for the treatment of intracranial aneurysms have been evolving over the past decade. In 1991, the advent of the Guglielmi detachable coil (GDC) (Target Therapeutics, Boston Scientific, Fremont, CA) significantly improved the endovascular treatment of these lesions by means of a reliable, electrolytically detachable, coiling system (1, 2). Embolization with GDCs is increasingly used for the treatment of intracranial aneurysms. The most frequent and serious complications of this technique are ischemic lesions due to thrombosis of the aneurysm's parent artery or to embolic phenomena from the partially thrombosed aneurysm. Thromboembolic events, including transient ischemic attack and stroke, have been reported to occur in 2.5% to 28% of cases (3–7). These ischemic complications occur more often during the procedure or within a few hours after treatment (8). In addition, some authors (9), using a transcranial Doppler sonographic monitoring system, have reported the occurrence of microemboli distal to endovascularly treated cerebral aneurysms, even in asymptomatic patients.
To our knowledge, MR imaging findings in patients treated with GDCs for an intracranial aneurysm have not yet been reported.
Newly developed MR techniques, including fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted sequences, can delineate ischemic tissue with a very high sensitivity (10–12). These pulse sequences are currently used to examine patients with acute neurologic symptoms of sudden onset (13–15). Consequently, in our institution for the last 6 months, diffusion-weighted MR imaging has been included in the management of patients with a cerebral aneurysm treated with GDCs to detect potential hyperacute ischemic lesions after embolization. The purpose of our study was to retrospectively evaluate the usefulness of diffusion-weighted imaging to detect changes in the brain of patients who underwent aneurysmal treatment with GDCs.
Methods
Between December 1998 and May 1999, 20 of 37 consecutive patients with a cerebral aneurysm scheduled for endovascular treatment with GDCs were studied with diffusion-weighted imaging before and after embolization. The remaining 17 patients did not undergo diffusion-weighted MR studies for the following reasons: the presence of an associated intracerebral hematoma (n = 4); severe neurologic status, necessitating intensive care (n = 8); or difficult access to or unavailability of the MR unit (n = 5). Of the 20 patients studied, five were men and 15 were women. Ages ranged from 23 to 75 years (mean age, 50 years). Twenty-one endovascular procedures were performed in 20 patients with one aneurysm each. Sixteen aneurysms were located in the anterior circulation (posterior communicating artery-internal carotid artery junction, n = 8; anterior communicating artery, n = 5; carotid-ophthalmic aneurysms, n = 2; middle cerebral artery, n = 1) and four were in the posterior circulation (top of the basilar artery, n = 1; posterior cerebral artery-basilar artery junction, n = 1; posterior cerebral artery at the P1–P2 junction, n = 1; posterior inferior cerebellar artery, n = 1). Except for the six aneurysms located on the midline, seven lesions were on the right side and seven were on the left. Seventeen aneurysms were small (<10 mm) and three were large (10–25 mm). Hemorrhage had occurred in 11 patients, of whom six were treated within the first 48 hours after bleeding (World Federation of Neurological Surgeons [WFNS], grades I–II) and five asymptomatic patients, referred from other institutions, were treated within 1 week (n = 1) or between 1 and 4 months (n = 4) after bleeding. In one of the latter five patients, hemorrhage was due to a contralateral aneurysm previously treated with GDCs. The other nine patients presented with a deficit of the third cranial nerve (n = 4), headache (n = 2), recurrent episodes of diplopia and vertigo (n = 1), or were asymptomatic (n = 2). One of the two asymptomatic patients had sustained cranial trauma and underwent cerebral angiography for a subarachnoid hemorrhage of the convexity; the other patient was found to have a familial aneurysm at screening CT angiography.
All 21 procedures were performed by an experienced interventional neuroradiologist. In all cases, GDCs were used to occlude the lesion. New 3D GDCs were used in three cases. During positioning of the coils, a balloon remodeling technique (16, 17) was performed in four patients. Only one patient with a large-diameter, wide-necked aneurysm underwent two endovascular procedures. All patients had general anesthesia and underwent heparinization. A bolus of 3000 to 5000 U heparin was given at the beginning of the procedure, followed by continuous infusion of 1000 U/hr to keep the activated clotting time at approximately five times the normal value. Heparinization was continued for up to 24 hours, allowing approximately twice the normal value. In selected cases, an intravenous bolus injection of aspirin during the procedure and/or long-term aspirin therapy was prescribed. Each patient was tested for motor, sensory, cranial nerve, and verbal responses before, immediately after, and the day after treatment.
MR studies were performed before treatment, 2 to 4 hours after treatment, and 24 to 48 hours after treatment. Each MR study included a fast FLAIR sequence with an imaging time of 4 minutes (5-mm axial interleaved sections, 10002/148 [TR/TEeff]; inversion time, 2200; matrix, 256 × 256; field of view, 24 × 24 cm; bandwidth, 32 kHz) and a diffusion-weighted sequence with an imaging time of 11 seconds (spin-echo multisection single-shot echo-planar sequences with a pair of diffusion gradients centered on a 180° pulse; axial section thickness, 6 mm; gap, 1.5 mm; TR, 2825; minimum TEeff; matrix, 96 × 64; field of view, 28 × 21 cm). Sixteen sections were acquired with a baseline T2 acquisition (b ≈ 0 s/mm2 [the b value is not strictly zero, as the gradients of the imaging sequence introduce some diffusion-weighting, but, for the practical purpose of our study, this contribution can be neglected]) and b = 1000 s/mm2 (diffusion gradient, G = 22 mT/m, active during 31 milliseconds). The diffusion gradients were successively and separately set in the three orthogonal directions and isotropic images were generated. Two experienced neuroradiologists independently reviewed all MR images. FLAIR and diffusion-weighted images were analyzed simultaneously. Observers searched for regions of hyperintensity on FLAIR and diffusion-weighted images. Apparent diffusion coefficient (ADC) maps were calculated using a dedicated software package (Functool, General Electric, Buc, France, 1997) by one neuroradiologist. The ADC maps were systematically calculated with circular regions of interest (ROIs) centered on diffusion-weighted signal abnormalities to calculate the mean ADC values. These were compared with the mean ADC values obtained in ROIs placed symmetrically in the contralateral hemisphere. In addition, whenever abnormal, the posttherapeutic mean ADC values were compared retrospectively with the pretherapeutic ADC values in the corresponding region. Follow-up FLAIR MR studies at 3 months were available for two patients.
Results
Occlusion of the aneurysm was obtained in 18 of 20 cases, and a small aneurysmal neck remnant was observed in the other two cases. No patient worsened clinically or incurred a deficit after treatment. After embolization, regression or disappearance of the symptoms was observed in four patients who had had paresis/paralysis of the third cranial nerve and in one patient who had had recurrent episodes of diplopia and vertigo. MR examination was successfully repeated three times for each patient, with only minor motion artifacts appearing on two studies in different patients. There was no discordance between the two observers in any of the MR assessments. No recent ischemic lesions were seen on any of the baseline diffusion-weighted or FLAIR images obtained before treatment. After treatment, early diffusion-weighted and FLAIR images showed no evidence of recent ischemic lesions in 18 patients. In these cases, follow-up MR studies at 24 to 48 hours were also normal.
In one patient, who had presented with headache and a right middle cerebral artery aneurysm, diffusion-weighted images obtained 2 hours after embolization showed an asymptomatic frontobasal hyperintense signal with a drop in ADC values (mean, 0.71 10−3 mm2/s, 85% of contralateral ADC value, 81% of pretherapeutic ADC value), corresponding to a clinically silent acute ischemic lesion. This lesion was barely visible on the corresponding FLAIR images. At the MR examination performed 24 hours after treatment, diffusion-weighted images confirmed the ischemic lesion (mean ADC, 0.68 10−3 mm2/s), which was also clearly observed on the corresponding FLAIR images (Fig 1). The patient remained asymptomatic and was discharged from the hospital 2 days later.
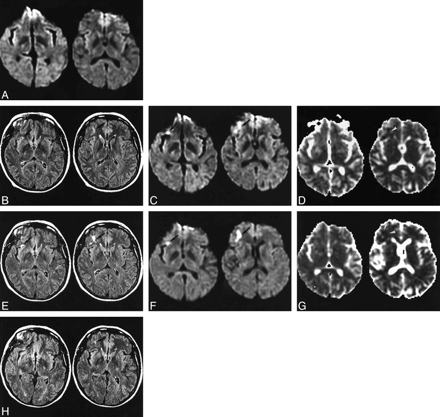
48-year-old woman with an aneurysm of the right middle cerebral artery trifurcation treated by GDCs.
A, Before treatment, diffusion-weighted images (2825/92.6/1) appear normal.
B–D, Two hours after embolization, FLAIR images (10002/148/2200/1) (B) show a slight right frontobasal hyperintense signal (arrows). Diffusion-weighted images (2825/92.6/1) (C) show a small right basal hyperintense lesion, caused by a recent ischemic event (arrows). On ADC maps (D), ADC values are decreased relative to contralateral hemisphere and displayed as an area of dark signal (arrowhead).
E–G, Twenty-four hours after embolization, the contrast of the lesion (arrows) has increased on FLAIR (10002/148/2200/1) (E) and diffusion-weighted (F) (2825/92.6/1) images, confirming the ischemic lesion. Values on ADC maps (G) are further decreased in the right frontobasal ischemic lesion (arrowheads).
H, Follow-up FLAIR images at 3 months (10002/148/2200/1) show a very small residual frontobasal hyperintense lesion (arrows).
In another patient with a subarachnoid hemorrhage (WFNS, grade II) caused by a posterior communicating artery aneurysm (treated with GDCs within 48 hours after bleeding), four silent lesions (three supratentorial and one infratentorial) were observed on diffusion-weighted images obtained 2 hours after embolization. Mean ADC values were decreased (0.63 10−3 mm2/s) only in the larger of the lesions as compared with the contralateral hemisphere (71%) and with pretherapeutic ADC values (75%). These silent lesions were not all located in the vascular territory of the aneurysm's parent artery. All lesions were seen better on diffusion-weighted images obtained 24 hours after embolization, when a fifth large lesion appeared in the posterior fossa on the left. Mean ADC values were decreased in all lesions except in a small left frontal lesion (Fig 2). These lesions were not visible on the FLAIR images obtained 2 hours after embolization but became visible on those obtained 24 hours later, including the fifth, more recent, lesion. The patient showed no clinical changes after treatment.
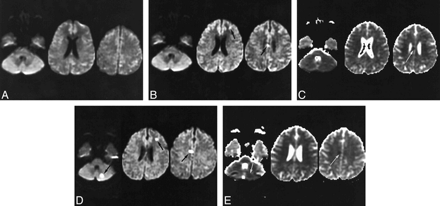
37-year-old woman with an aneurysm of the left posterior communicating artery who presented with subarachnoid hemorrhage (WFNS, grade II).
A, Before embolization, diffusion-weighted images (2825/92.6/1) show foci of slight hyperintense signal due to the presence of subarachnoid hemorrhage.
B and C, Two hours after embolization, four silent small lesions were observed on diffusion-weighted images (2825/92.6/1) (only two of the four lesions are shown [arrows, B]). An area of hypointense signal is seen on ADC maps (arrow, C), with decreased ADC values.
D and E, Twenty-four hours after embolization, lesions are more visible on diffusion-weighted images (D), with a fifth lesion (arrow) apparent in the posterior fossa. On ADC maps (E), decreased ADC values are seen as dark areas for all lesions (arrows), except for the small left frontal lesion. These silent lesions are not all related to the aneurysm's parent artery, and their origin remains uncertain.
In these two patients, endovascular treatment was performed with standard GDCs and without the remodeling technique. The follow-up FLAIR images at 3 months showed a very small residual frontobasal hyperintense lesion in the first case (Fig 1). In the second case, the only visible lesion was the one in the posterior fossa. No new lesions were observed on follow-up MR studies in either patient.
Discussion
GDCs have been used since 1991 and represent a major technical advancement in the endovascular treatment of intracranial aneurysms (18). Technical complications associated with the use of GDCs include aneurysmal perforation and rupture, parent artery occlusion, cerebral embolisms, coil rupture, coil migration, and vasospasm. Complication rates of endovascular treatment of aneurysms appear to be in the range of 3% to 5% morbidity (with an immediate morbidity rate of 9%) and 1.5% to 5% mortality (3–6). The most frequent and serious complications of endovascular treatment with GDCs are ischemic lesions caused by thromboembolic phenomena.
In a prospective multicenter clinical study of 403 patients with a ruptured aneurysm, unintentional parent artery occlusion was reported with a frequency of 3% and cerebral embolism with a frequency of 2.5% at angiography (5). Clinical thromboembolic complications, including transient ischemic attack and stroke, have been reported to occur in 2.5% to 5% of cases (3–6, 19), even after uncomplicated and uneventful embolization procedure (7, 8). However, some authors (7) have suggested that thromboembolic events related to GDC treatment may be more common than what has been reported in the literature. In their experience, this rate has been 28%, with persisting deficits in 5%. Some authors (9) have investigated the frequency of microemboli distal to cerebral aneurysms before and after coil embolization by using a transcranial Doppler sonographic monitoring system. After coil embolization of the aneurysm, microemboli were detected in 31% of cases, including asymptomatic patients, but occurred more often in patients who suffered from cerebral ischemia.
Thromboembolic events occur more often during the procedure or within the first hours after treatment. If complications occur during endovascular treatment of an intracerebral aneurysm, then immediate superselective pharmacologic thrombolysis appears to be a safe and efficient therapy that increases the rate of recanalization and improves the chance of a better clinical outcome (8). After endovascular treatment, the challenge is to detect the thromboembolic event early enough to closely monitor the patient and to set up a prompt endovascular and/or systemic therapy. In addition, thromboembolic complications can be initially silent, becoming symptomatic only when the infarct enlarges. These ischemic lesions may be undetected on initial CT scans and on conventional MR studies obtained during the first 12 hours after onset (7, 12–14), a period during which stroke therapies are most likely to be effective (20). Studies of diffusion-weighted imaging in humans suffering from acute stroke have confirmed the superiority of this technique relative to T2-weighted imaging within the first few hours after stroke onset (13–15, 21, 22). This recently validated MR technique appears to be a considerable improvement in the diagnosis and treatment of patients with acute neurologic symptoms. Consequently, to detect potential hyperacute ischemic lesions after embolization, we have included diffusion-weighted MR imaging in the management of patients with a cerebral aneurysm treated with GDCs. These new MR techniques have previously been proposed to evaluate early ischemic changes after carotid endarterectomy (23, 24). In addition, some authors (25) have recently used diffusion-weighted MR imaging to examine patients after diagnostic and interventional angiography. However, that series did not include patients treated for cerebral aneurysms.
The purpose of our study was to retrospectively evaluate the usefulness of diffusion-weighted MR imaging to detect ischemic brain changes in patients who had undergone endovascular treatment with GDCs for an aneurysm. For this preliminary study, we excluded patients who were under intensive care because of the practical difficulty in performing MR imaging in these circumstances. Although some authors (26) have reported that the presence of parenchymal hemorrhage does not obscure the interpretation of diffusion-weighted images when looking for acute ischemic lesions, the exact sensitivity of diffusion-weighted images for the detection of ischemic stroke is still unclear when an associated cerebral hematoma exists. For this reason, we also excluded patients with an associated intracerebral hematoma.
The entire MR examination, with a total acquisition time of less than 5 minutes, was repeated three times for each patient. MR studies were successful in all cases, although some patients were experiencing subarachnoid hemorrhage. This suggests the feasibility of the technique in this clinical context. The decision to perform an MR examination before treatment was based on the fact that subarachnoid hemorrhage can produce a hyperintense signal on diffusion-weighted images that could be mistaken for small cortical infarct(s). The baseline images were useful for identifying subarachnoid signal changes on diffusion-weighted images in the case of massive hemorrhage. In addition, any recent ischemic lesions due to embolic fragments that migrated from the aneurysm before treatment, vasospasm, or ischemic events of other origin, especially in the elderly, should be detected before treatment in order to distinguish them from thromboembolic complications resulting from treatment.
Immediate posttreatment MR examinations were scheduled between 2 and 4 hours after treatment to allow early detection of thromboembolic events related to embolization. Since contrast-to-noise ratio increases on diffusion-weighted images during the first 48 hours after stroke (27, 28), a follow-up MR study was scheduled between 24 and 48 hours after treatment. This examination was performed to detect small ischemic lesions undetected on the earlier posttherapeutic MR study and to detect delayed ischemic events that may occur after the first hours after treatment.
The 24- to 48-hour MR study did not increase diagnostic accuracy in any case in which the 2- to 4-hour study was negative. It was useful for the follow-up of the two patients who had positive findings on the 2- to 4-hour study to check for the extension and number of ischemic lesions. Our imaging protocol has since been simplified, and the 24- to 48-hour MR study is no longer performed in asymptomatic patients with normal findings on the 2- to 4-hour examination. ADC values were decreased in most of the lesions seen on diffusion-weighted images, consistent with the ischemic nature of the lesion. However, ADC values were normal in the very small lesions, especially on the early MR examination. Thus, ADC calculation does not seem to be a reliable tool for evaluation of very small lesions. Since hemodynamically weighted imaging seems to offer the ability to identify early progression of ischemic injury and tissue at risk (21, 28, 29), it will be included in our MR protocol in the future.
In most patients, no lesions were observed on diffusion-weighted MR studies obtained after GDC treatment, including in those patients in whom a remodeling technique had been used. These results could be biased by the fact that we only studied patients with a relatively good clinical status. Nevertheless, except for angiographic vasospasm, more severe clinical conditions, necessitating intensive care, should not affect the feasibility of endovascular treatment with GDCs. Consequently, the frequency of ischemic lesions after GDC embolization is probably not underestimated. Two of 20 patients had abnormal MR findings on diffusion-weighted images after embolization. In the first case (Fig 1), the frontobasal ischemic lesion was probably due to a distal embolic event, since adjacent branches were permeable and normal at angiography. This was the only patient who had a middle cerebral artery aneurysm, which is usually not a good indication for endovascular treatment. In the second case, a patient with a posterior communicating artery aneurysm (Fig 2), multiple silent lesions were observed, but not all were located in the vascular territory of the aneurysm's parent artery. Their origin is uncertain. We could speculate about multiple emboli or transient vasospasm phenomena; however, the angiography and embolization were uncomplicated, and no angiographic vasospasm was observed at the time of embolization. The additional lesion that appeared in the posterior fossa on the delayed MR study does not seem to be related to the GDC procedure. It could again be explained by a successive event, such as vasospasm.
In a single recent report concerning detection of early ischemic injury due to vasospasm after subarachnoid hemorrhage (29), diffusion-weighted images showed small ischemic lesions in six patients with symptomatic and angiographic vasospasm, and diffusion-weighted imaging findings were normal in two asymptomatic patients. An important aspect to be considered is that complications can also arise from the angiographic phase of the GDC therapeutic procedure itself, especially in elderly patients or in those with a vascular risk profile (25). In patients with transient ischemic attacks or previous strokes, a permanent neurologic angiographic complication rate of 0.5% to 1% has been reported (30, 31). In addition, some authors (31, 32) have reported that transcranial Doppler sonography performed during diagnostic angiography frequently shows the presence of numerous cerebral microemboli caused by introduced air. However, these microemboli are usually clinically silent and are not associated with signal changes on T2-weighted MR images (31).
Regarding the frequent occurrence of microemboli during angiography and coil embolization (9, 31, 32), our results suggest that most microemboli do not cause cerebral ischemic lesions or that they only cause very small, clinically silent lesions, undetected on diffusion-weighted images. In a recent study (25), the presence of silent embolism after diagnostic and interventional angiography was evaluated using diffusion-weighted MR imaging. The studies showed bright lesions consistent with embolic events in 17 (26%) of 66 patients after diagnostic angiography and in six (18%) of 34 patients after an interventional angiographic procedure. These data are not comparable with our results, because the authors did not study patients treated for cerebral aneurysms. There were no new neurologic deficits after any angiographic procedure in this series. In both our pathologic cases, the small ischemic lesions remained clinically silent and required no change in the standard therapy after embolization. We encountered no important ischemic events in our series, so we cannot conclude whether diffusion-weighted imaging really improves the management of patients with ischemic complications from GDC therapy. In any case, diffusion-weighted images do allow an excellent evaluation of brain parenchyma after embolization that is particularly useful when clinical examination is difficult, such as after general anesthesia.
Conclusion
These preliminary results suggest that diffusion-weighted MR imaging is a potentially useful tool for monitoring patients after endovascular treatment of cerebral aneurysms. This examination could permit early detection of ischemic complications, such as extension of thrombosis and embolic phenomena, leading to their early treatment. Although small asymptomatic lesions can be observed on the posttherapeutic diffusion-weighted images, the exact prevalence of these lesions should be evaluated in a larger series.
Footnotes
1 Presented in part at the annual meeting of the American Society of Neuroradiology, San Diego, May 1999.
↵2 Address reprint requests to Alessandra Biondi, MD, Department of Diagnostic and Therapeutic Neuroradiology (H. Fischgold), Bâtiment Babinski, Pitié-Salpêtrière Hospital, 47–83 Boulevard de l'Hôpital, 75651 Paris, Cedex France.
References
- Received July 26, 1999.
- Copyright © American Society of Neuroradiology