Abstract
Summary: We describe our initial clinical experience using the newly available self-expanding, Nitinol, shape-memory–, alloy-recoverable–technology (SMART) stent in treating carotid artery occlusive disease. Five stents were used in four carotid arteries in four consecutive patients with carotid stenosis of at least 70%. Technical success (<20% residual stenosis) was achieved in all cases. No procedural complications specifically related to use of the SMART stent were encountered. All patients remained symptom-free, with no evidence of transient ischemic attacks or new strokes during an average follow-up period of 6 months. Excellent performance of the SMART stent for the endovascular treatment of carotid artery stenosis has been shown based on our early experience. Validation with greater numbers and longer-term follow-up is required. The specific technical characteristics, potential advantages, and disadvantages of this stent are discussed and compared with other currently used carotid artery stents.
Carotid endarterectomy is currently considered the standard of reference for treatment of atherosclerotic carotid occlusive disease. This has been validated by multiple, large, randomized, controlled trials, proving its efficacy over medical therapy. These trials have shown that endarterectomy confers a notable benefit for patients with symptomatic carotid stenosis of 70% to 99% (1–3), with lesser, albeit significant, degrees of benefit in symptomatic lesions of 50% to 69% (4) and asymptomatic stenosis of at least 60% (5). During the past several years, however, carotid artery stent-supported angioplasty has emerged as a potential therapeutic alternative to carotid endarterectomy for the treatment of carotid artery occlusive disease. This is based on numerous, large, retrospective studies that have shown rates of technical success (procedure-related morbidity, mortality, and restenosis rates that compare favorably with those of carotid endarterectomy) (6–16). Early studies primarily used balloon-expandable stents, such as the Palmaz stent (Johnson & Johnson Interventional Systems Co., Warren, NJ). Subsequent reports of a 2% to 16% deformity and crush rate (17, 18), however, have led to greater use of self-expanding, crush-resistant types, such as the Wallstent (Schneider, Minneapolis, MN). The shape-memory–alloy-recoverable–technology (SMART) stent (Cordis Endovascular, Miami Lakes, FL), a new self-expanding stent composed of Nitinol, has recently become available for clinical use in the United States. The term Nitinol is derived from (Ni)ckel, (Ti)tanium, and (N)aval (O)rdinance (L)aboratories of the United States Government, where Nitinol was first developed during the 1960s. We review our early clinical experience using this stent for the endovascular treatment of carotid occlusive disease.
Description of Technique
Patients received orally administered enteric-coated aspirin (325 mg/day) and clopidogrel (75 mg/day), commencing 3 days before the procedure. All procedures were begun with the patients under conscious sedation; however, conversion to general anesthesia was required in two cases because of patient agitation and excessive movement. After the insertion of a 9F groin sheath into the common femoral artery, a baseline activated clotting time was obtained.
Complete diagnostic cerebral angiography, including intracranial views and assessment of the collateral circulation, was performed in all cases. Quantitative measurements of the normal common and internal carotid artery diameters and length of stenosis were obtained using an inbuilt digital calibration system (Toshiba, Tustin, CA). Next, all patients received IV administered heparin bolus (70−100 units/kg) to obtain an activated clotting time value of 2 to 2.5 times baseline or greater than or equal to 250 seconds. A continuous heparin infusion of 15 to 20 U/kg/hr was used in three of four cases for the duration of the procedure. An IV administered bolus of abciximab (25 mg/kg) and then a continuous infusion (10 mg/kg/hr) for a period of 12 hours was used in the fourth case. Abciximab is a platelet glycoprotein IIb/IIIa inhibitor that has been shown to decrease mortality and morbidity in a number of coronary stent studies (19). A 9F guiding catheter was placed into the common carotid artery over a 0.035-in, 300-cm exchange-length wire positioned in the ipsilateral external carotid artery.
The stenosis was initially crossed under digital roadmap guidance with a 2.3F microcatheter (Rapid Transit, Cordis Endovascular) and a 0.014-in guidewire (Transend; Scimed, Maple Grove, MN), which was then exchanged for a 300-cm, 0.014-in exchange-length guidewire (Balance; Advanced Cardiovascular Systems Inc., Santa Clara, CA). Before balloon inflation, 0.1 to 0.2 mg of glycopyrrolate was IV administered to prevent reflex bradycardia or asystole. Predilation using a 3.5- to 4.0-mm diameter, 18-mm-length balloon angioplasty catheter (Titan, Cordis Endovascular) was then performed in three of four cases. A digital roadmap image was obtained, and the stent delivery catheter was then advanced over the immobilized guidewire. After stent deployment, balloon angioplasty within the stent, performed using a high-pressure (12- to 20-atm), semicompliant balloon (Jupiter, Cordis Endovascular), was done in three of four cases.
Intravenous heparinization was continued overnight in three patients. Heparinization was then allowed to taper physiologically. Femoral sheaths were removed using an external compression device (FemoStop; Radi Medical Systems, Uppsala, Sweden) when the partial thromblastin time had returned to normal. In the patient receiving abciximab infusion, the femoral sheath was removed 4 hours after the procedure by using an external compression device (FemoStop) when the activated clotting time had returned to baseline. In all patients, oral clopidogrel (75 mg/day) was continued for 6 weeks and enteric-coated aspirin (325 mg/day) was continued indefinitely. Carotid duplex sonography was performed at the 6-month follow-up examination.
Case 1
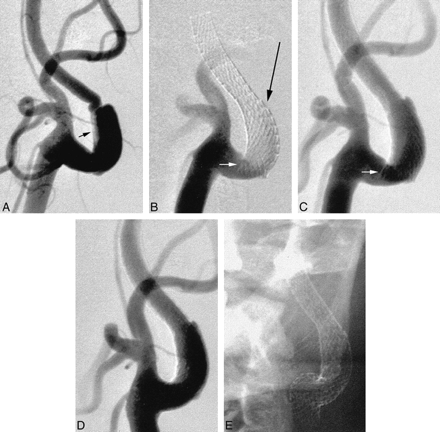
A 50-year-old right-handed man presented with acute onset of severe headache, nausea, vomiting, and abdominal pain. A clinical examination revealed meningismus, gait instability, horizontal diplopia, and severe hypertension (mean arterial blood pressure, 160 mm Hg). These neurologic symptoms resolved during the next few days. A subsequent diagnostic workup revealed a nonaneurysmal subarachnoid hemorrhage with diffuse intracranial vasospasm, a proximal left internal carotid artery dissection with severe luminal stenosis and pseudoaneurysm formation, and a right renal artery dissection with severe stenosis and renal parenchymal infarction. Revascularization of the left internal carotid artery stenosis was performed before open surgical revascularization of the right renal artery stenosis because of the risk of thromboembolism associated with abdominal surgery when anticoagulation was ceased. An 8-mm-diameter × 40-mm-length SMART stent was deployed across the stenotic segment and pseudoaneurysm. The caudal portion of the stent, however, was unable to conform to the carotid bulb contour, resulting in partial luminal obstruction. This was resolved by deployment of a 10-mm-diameter × 20-mm-length SMART stent across the carotid bifurcation, inferiorly overlapping the first by approximately 5 mm (Fig 1). Pre- or poststent deployment balloon dilation was not performed. There were no procedural complications, and the patient achieved an uneventful postprocedural recovery. Six weeks later, he underwent successful surgical repair of his right renal artery dissection.
Anteroposterior views of the left carotid artery bifurcation.
A, Acute dissection involving the proximal internal carotid artery. The true lumen is severely narrowed (arrow), with early pseudoaneurysm formation.
B and C, Position of the first SMART stent, the inferior margin of which projects across the internal carotid artery lumen (small arrow). The long thin arrow indicates where the catheters were temporarily caught in the stent interstices (see Discussion).
D and E, Final appearances after deployment of a second SMART stent, inferiorly and overlapping the first. The second stent crosses the external carotid artery origin, which nonetheless continues to opacify normally.
Case 2
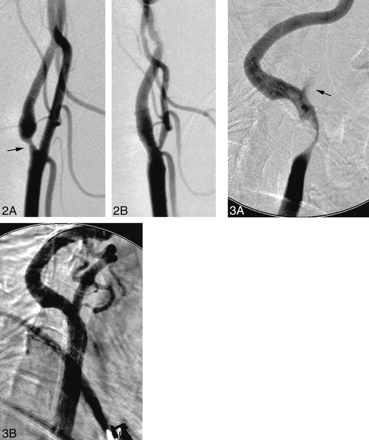
A 44-year-old right-handed woman with a history of a severe whiplash injury presented with sudden onset of mild right hemiparesis and expressive dysphasia. A CT scan revealed a small, left middle cerebral artery territory infarction. A diagnostic workup revealed a severe stenosis of the proximal left internal carotid artery (approximately 70%) with a 12-mm-diameter aneurysm of the ipsilateral precavernous segment. A regimen of warfarin was subsequently commenced. The patient achieved a good neurologic recovery apart from mild, persisting, right central facial nerve palsy and word-finding difficulty. Warfarin was discontinued 3 days before the carotid stenting procedure. Predilation of the carotid stenosis was performed using a 4-mm-diameter × 18-mm-length balloon (Titan). Next, an 8-mm-diameter × 20-mm-length SMART stent was deployed across the carotid stenosis. Postdilation was performed using a 5.5-mm-diameter × 20-mm-length balloon (Jupiter) (Fig 2). Endovascular coil embolization of the precavernous segment aneurysm was then successfully performed. There were no procedural complications, and the patient achieved an uneventful postprocedural recovery.
Lateral views of the left carotid artery bifurcation.
A, Approximately 70% short-segment, circumferential, atherosclerotic stenosis of the internal carotid artery origin (arrow).
B, Appearances after deployment of a SMART stent, which crosses the origin of the external carotid artery. Note stenosis of the external carotid artery origin as a consequence of stenting across the carotid bifurcation. This is usually of no clinical significance.
fig 3. Lateral views of the right carotid artery bifurcation.
A, Severe (approximately 90%), long-segment, atherosclerotic stenosis of the common and internal carotid arteries. There is minimal opacification of the external carotid artery (arrow).
B, Appearances after deployment of a SMART stent within the common carotid artery.
Case 3
A 78-year-old, obese, right-handed woman with a history of a left pontine stroke with residual mild right-sided weakness and hypertension presented with an acute right middle cerebral artery stroke, with moderate left-sided weakness and neglect and a left homonymous hemianopia. A regimen of heparin infusion was commenced, and the patient achieved good neurologic recovery during the next several days. A carotid duplex Doppler examination revealed a severe stenosis of the right internal carotid artery. Diagnostic angiography revealed a severe (approximately 90%) stenosis of the distal right common and proximal internal carotid artery. There was no filling of the ipsilateral external carotid artery. The contralateral carotid artery was narrowed by approximately 60%. Collateral supply to the right anterior cerebral circulation was mainly from the right posterior communicating artery. Predilation of the right carotid stenosis was performed using a 4-mm-diameter × 20-mm-length balloon (Savvy, Cordis Endovascular) and then an 8-mm-diameter × 40-mm-length SMART stent was deployed. Postdilation was performed using a 6-mm-diameter × 20-mm-length balloon (Jupiter) (Fig 3). Postprocedurally, the patient developed a right groin hematoma with a fall in the hematocrit value requiring transfusion of two units of packed RBC. There were no other complications, and the patient was neurologically stable.
Case 4
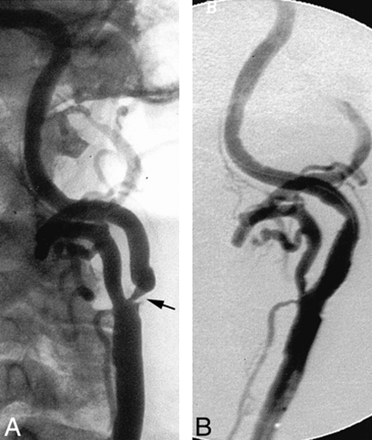
An 85-year-old man presented with a history of hypertension and coronary artery disease. An asymptomatic severe stenosis (approximately 90%) of the left internal carotid artery was detected by carotid duplex Doppler examination during workup for possible coronary artery revascularization. Predilation of the carotid stenosis was performed using a 4-mm diameter × 18-mm length balloon (Titan). An 8-mm-diameter × 20-mm-length SMART stent was deployed across the stenosis. Next, postdeployment balloon angioplasty was performed using a 5.5-mm-diameter × 20-mm-length balloon (Jupiter) inflated to 20 atm (nonconstrained diameter of 5.8 mm) (Fig 4). There were no procedural complications. The patient suffered two episodes of acute urinary retention, precipitating acute pulmonary edema approximately 36 to 48 hours postprocedurally. Laboratory values indicated a small myocardial infarction without associated ECG changes. Urinary retention was secondary to moderate hematuria, possibly precipitated by abciximab. The patient subsequently achieved an uneventful recovery and remained neurologically intact.
Ipsilateral oblique views of the left carotid artery.
A, Severe short-segment, circumferential, atherosclerotic stenosis of the internal carotid artery origin (arrow).
B, Appearances after deployment of a SMART stent within the internal carotid artery.
Results
Technical success (<20% residual lumen) was achieved in all cases. All atherosclerotic carotid stenoses (n = 3) required postdeployment angioplasty within the stent for residual stenosis. The periprocedural 30-day rate of any stroke and death was 0%. One patient suffered a postprocedural small myocardial infarction and another a groin hematoma. Clinical follow-up of these patients (average follow-up period of 3 months) revealed no evidence of transient ischemic attacks or new strokes. All patients remained at their neurologic baseline. Longer-term clinical or imaging follow-up is not yet available.
Discussion
Early trials of carotid artery stenting primarily used balloon-expandable stents, such as the stainless steel Palmaz stent (Johnson & Johnson Interventional Systems Co.) and the Strecker stent (Medi-tech, Watertown, MA), which can usually be positioned with greater accuracy than can self-expandable types (6–9, 11–13). Follow-up imaging, however, revealed cases of Palmaz stent compression and collapse (6, 10, 17, 18). Mathur et al (17) reported Palmaz stent collapse in 11 (16%) of 70 patients at 6-month angiographic follow-up, attributing this to probable external compression. Furthermore, carotid Doppler sonography performed retrospectively in seven patients with stent compression was only 29% sensitive. Compression of balloon-expandable stents has been reported to be a significant cause of restenosis in the superficial femoral arteries and in hemodialysis grafts (20). Subsequently, use of self-expandable stents, such as the Wallstent (Schneider), has become more frequent (14–16, 21). In a 1998 global survey, Wholey et al (18) reported on 3033 endovascular carotid stents that had been placed. Balloon-expandable Palmaz stents were used in 47% of the cases, self-expandable Wallstents in 46%, and other stents, including Strecker, Integra (Medi-tech), Symphony (Medi-tech), and SMART stents, in the remaining 7%. Twenty-eight stent deformations occurred, and they occurred exclusively with the Palmaz stainless steel stents (2%) (18).
The SMART stent is composed of Nitinol, a nickel-titanium metallic alloy. Nitinol possesses the property of shape memory. This refers to the ability of Nitinol to assume a predetermined shape when heated above a preset transition temperature. Heating induces a phase transformation in the alloy. The atomic arrangement changes from a Martensite phase, in which the material is very malleable, to an Austenite phase, in which the material exhibits a high degree of strength and superelasticity (22, 23). Superelasticity affords Nitinol a high degree of flexibility, kink, and fatigue resistance. The preset transition temperature of the SMART stent is set at 26°C to 32°C. Uncovering the stent within the carotid artery exposes it to body temperature, whereupon it instantaneously assumes its memorized shape.
The current SMART stent-delivery system requires the use of a 7F sheath or 9F guiding catheter. Injecting contrast medium through a 9F guiding catheter containing the stent delivery catheter is difficult. If, however, a 0.014-in guidewire is used, it is possible to obtain a satisfactory angiographic or digital roadmap image by injecting through the delivery catheter lumen using a Tuohy-Borst type rotating hemostatic valve (Big Easy; Microvena, White Bear Lake, MN). We have observed the stiffness of the SMART stent delivery catheter to be equivalent to that of the Wallstent. Navigating prominent arterial bends can be difficult because of the catheter rigidity. Navigation of such acute arterial bends can occasionally be assisted by manually shaping the tip of the delivery catheter. Rigidity of the delivery catheter may also result in straightening of arterial curves. This can potentially render digital roadmaps or bony landmarks delineating the desired stent position inaccurate.
In our experience, the positional accuracy of deployment of the SMART stent is generally superior to that of the Wallstent. The SMART stent foreshortens by less than 8% at its hub end. The phenomenon of “jumping,” which refers to the propensity for abrupt forward movement of Nitinol stents from the delivery catheter during deployment, can be overcome by ensuring that all of the redundancy has been removed from the stent delivery catheter before deployment and by slowly and smoothly retracting the outer constraining sleeve. After deployment in Case 4, we experienced minor difficulty in withdrawing the stent delivery catheter through the stent. This was resolved by resheathing the delivery catheter tip by manual advancement of the outer sleeve.
The SMART stent possesses segmented-hoop geometry with very short individual segments (Fig 5). This segmented geometrical design results in individual stent hoops behaving fairly independently of each other. The Wallstent possesses a braided geometry in a tubular mesh configuration. The braided wires are wrapped the entire length of the stent and are not independent of each other. The geometry of the SMART stent in association with the elastic properties of Nitinol results in important behavioral differences between the SMART stent and the Wallstent. In our experience, the SMART stent is better able to adapt to the native vessel contour successfully, thus minimizing vessel straightening, which is a characteristic referred to as conformability (Fig 1). Therefore, the propensity for producing vessel straightening with relocation of arterial redundancies and bends to the proximal and distal ends of the stent is reduced. This phenomenon can result in kinking of the carotid artery at the stent margins, although in our experience, these nouveau kinks have generally not been flow-limiting. Unlike the Wallstent, once deployment has commenced, the SMART stent is not reconstrainable.
Photograph of the SMART stent shows segmented geometry and flared margins.
Although the SMART stent offers improved conformability, it is still recommended that the stent margins be positioned within relatively straight segments of the carotid artery, where possible. In Case 1, the caudal end of the stent was positioned within an acutely rounded portion of the vessel. The inferior portion of the stent was unable to conform to the carotid bulb contour, resulting in protrusion of the stent across the carotid lumen (Fig 1). This was resolved by deployment of an inferiorly overlapping SMART stent. This required re-entry of the stent lumen using a triaxial system consisting of a 4F catheter containing a 2.3F microcatheter primed with an 0.014-in guidewire. The ends of the current SMART stent are flared, and there was no difficulty in reentering the stent lumen. Approximately half-way along the stent, however, at a point of increased curvature, the tips of both catheters were arrested by the stent interstices (Fig 1, arrow). We have not previously encountered this problem when using the Wallstent. This is partly explained by the proclivity of the Wallstent to maintain a linear rather than curvilinear configuration and by its braided rather than segmented geometry.
In terms of stent sizing, we selected a stent diameter 1- to 2-mm larger than the largest opacified vessel diameter the stent would need to oppose. This method of sizing stent diameter is similar to that for the Wallstent. Undersized Nitinol stents will continue to exert a chronic outward radial force on the arterial wall (22). Whether shape-memory stents deployed within the carotid artery will eventually expand to their preset diameter with time is not known. This is relevant when stenting across the carotid bifurcation where significant over-sizing of the internal carotid artery diameter may occur. This also has implications regarding the need for postdeployment stent angioplasty. If after deployment there is good wall apposition without residual stenosis, angioplasty may theoretically be unnecessary because an oversized SMART stent will potentially continue to expand or at least exert a persistent radial force. Postdilation of residual stent stenosis is our current practice. This was required in all three cases of atherosclerotic stenosis. After deployment, Nitinol stents offer the advantage over stainless steel stents of crush recoverability or “springlike” behavior. If an external force compresses or deforms the SMART stent, it will immediately reassume its expanded shape at the time of removal of the external stress. This is particularly advantageous in superficial or exposed arteries, such as the carotid artery, in which significant crush rates associated with balloon-expandable stents have previously been reported (6, 17, 18). In our experience, the radiovisibility of the SMART stent is equivalent to, although not necessarily superior to, that of the Wallstent.
We encountered two complications in this small series consisting of a groin hematoma (case 3) and a postprocedural, small, non–Q wave myocardial infarction (case 4). Although both patients achieved uneventful recoveries, these complications prolonged their inpatient stay. The use of the FemoStop compression device in an obese patient with a 9F groin sheath likely predisposed the patient to a groin hematoma in case 3. Future reduction in the diameter of stent-delivery catheters requiring smaller caliber groin sheaths and newer percutaneous suture delivery devices may help to reduce the incidence of this complication, particularly in obese patients. The postprocedural myocardial infarction occurred in an elderly patient with coronary artery disease. In the North American Symptomatic Carotid Endarterectomy Trial, the overall incidence of periprocedural myocardial infarction was 1.2% (24). Patients with a history of angina pectoris, myocardial infarction, or hypertension were at a significantly higher risk. In the Carotid and Vertebral Artery Transluminal Angioplasty Study, however, myocardial ischemia at the time of procedure did not occur in the angioplasty group (25). Despite these results, it remains unclear whether carotid stenting is associated with a significantly lower rate of periprocedural myocardial infarction compared with endarterectomy. Careful intra- and postprocedural management of hemodynamic parameters and fluid status may help to reduce the likelihood of this complication.
Conclusion
Our early clinical experience has shown excellent performance of the SMART stent for treatment of carotid artery stenosis. Technical success was achieved in all cases. No significant technical problems or procedural complications directly related to the use of the SMART stent were encountered. Long-term follow-up is required to assess the patency rates of this stent in comparison with those of other currently used stents, such as the Wallstent for carotid artery occlusive disease.
Footnotes
↵1 Address reprint requests to Randall T. Higashida, MD, Division of Interventional Neurovascular Radiology, University of California San Francisco Medical Center, 505 Parnassus Avenue, Room L-352, San Francisco, CA 94143.
References
- Received July 20, 1999.
- Copyright © American Society of Neuroradiology