Abstract
Summary: Subependymal heterotopia consist of gray matter nodules along the lateral ventricular walls and are associated with epilepsy and other cerebral malformations. Some cases have an X-linked inheritance, and early antenatal diagnosis of affected fetuses is important for appropriate management. We present a case of heterotopia diagnosed by sonography and MR imaging at 23 weeks' gestation and discuss the differential diagnosis, reviewing the evolution and imaging appearances of the germinal matrix and its implications for detection of heterotopia.
Subependymal heterotopia consist of clusters of disorganized neurons and glial cells that are located in close proximity to the ventricular walls (1). They are frequently associated with seizures and variable intellectual deficits (2−4). The underlying genetic lesion causing many familial cases of this condition has recently been determined (5), and with this knowledge, antenatal diagnosis becomes more important in affected families. Although sonography is the mainstay of fetal assessment, there has been considerable interest in the use of MR imaging as a complement to sonography for further characterization of fetal abnormalities (6−16).
The early antenatal diagnosis of subependymal heterotopia is potentially difficult because of practical problems concerning motion (both of the fetus and of the maternal abdominal wall) and obtaining adequate resolution of the small fetal brain. Another potential problem is the presence of the periventricular germinal matrix, which is prominent in early brain development and does not involute until the 26th week (17). Before that, one is faced with the potential difficulty of distinguishing subependymal heterotopic gray matter from normal germinal matrix on MR images. We report a case in which bilateral periventricular nodular heterotopia were identified on both sonograms and MR images at 23 weeks' gestation.
Case Report
A 42-year-old pregnant woman with no relevant medical history was referred for detailed fetal morphologic sonographic assessment after previous routine sonography performed at another hospital suggested the presence of Dandy-Walker malformation. The fetal sonogram was performed at 23 weeks' menstrual age and revealed an anechoic space in the posterior fossa. The cerebellar vermis and fourth ventricle appeared normal, suggesting the diagnosis of a mega cisterna magna rather than Dandy-Walker malformation. In addition, the margins of the lateral ventricles were noted to contain a few small nodules protruding into the lateral ventricles (Fig 1A–B); in conjunction with the mega cisterna magna, these nodules were suspicious for gray matter heterotopia. No other abnormalities were shown.
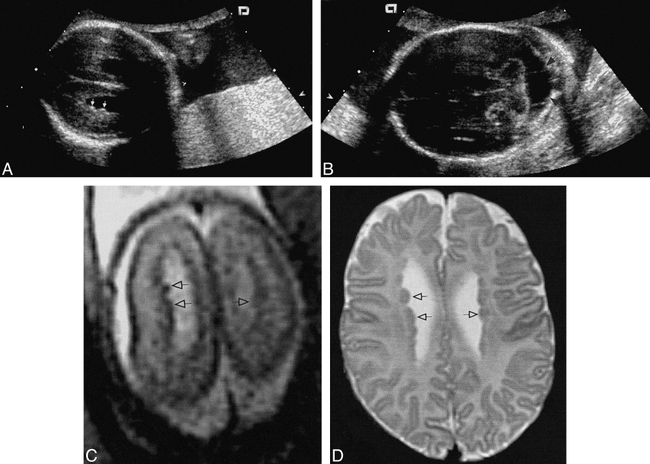
Images from the case of a 42-year-old pregnant woman who was referred for detailed fetal morphologic sonographic assessment after previous routine sonography suggested Dandy-Walker malformation.
A, Fetal sonogram obtained at 23 weeks' gestation. Axial image, obtained through the upper aspect of the lateral ventricles, shows an irregular ventricular wall with very small hyperechoic nodules along the lateral ventricular walls (arrows).
B, Fetal sonogram obtained at 23 weeks' gestation. Axial image, obtained through the posterior fossa, shows the large cisterna magna (arrows).
C, Axial single-shot fast spin-echo (21901/93/0.5) image shows the lateral ventricles of the fetal brain at 23 weeks' gestation. The lateral ventricular wall is slightly irregular because of the presence of tiny subependymal nodules isointense to the cortex that project into the ventricular lumen (arrows).
D, Axial spin-echo (3000/120) image, obtained 2 months after birth through the lateral ventricles at a level similar to that shown in C, shows multiple subependymal heterotopic nodules that are isointense to gray matter (arrows), corresponding to the nodules seen on the fetal MR image.
Fetal MR imaging was performed to evaluate the possibility of heterotopia further. Imaging was performed on a 1.5-T system using the body coil, and 4-mm half-Fourier single-shot fast spin-echo (21901/93/0.5, TR/TE/excitations) T2-weighted images were obtained in planes that were approximately sagittal, coronal, and axial with respect to the head of the fetus. Single-slice 5-mm T1-weighted gradient-echo (100/4/1) images with gradient spoilers were also obtained in three similar planes. No fetal or maternal sedation was administered. The T2-weighted MR images confirmed the presence of multiple small nodular subependymal foci of low signal, similar to the signal of gray matter, thought to represent nodules of heterotopic gray matter (Fig 1C). A large cisterna magna was confirmed, and no other posterior fossa or supratentorial malformation was shown. The pregnancy progressed uneventfully, and on a follow-up sonogram at 33 weeks' gestation, multiple small nodules were visible along the lateral ventricles, particularly along the frontal horns and bodies. This was more marked on the right side than on the left, and the frontal horns were enlarged.
The pregnancy remained uneventful, and a normal-appearing female neonate was delivered at term without difficulty. The neonatal period was clinically uneventful. An MR image of the head was obtained 2 months after delivery to confirm the diagnosis of heterotopia and to determine whether other anomalies not detected by fetal imaging were present. Sagittal spin-echo (600/11/1) 3-mm images, axial spin-echo (3000/60,120/1) 4-mm images, coronal 3D Fourier-transformed gradient-echo (35/7/1) 1.5-mm images, and fast spin-echo (3500/108) 3-mm images were obtained. The images revealed multiple round-to-ovoid subependymal nodules in the walls of the lateral ventricles, consistent with subependymal heterotopia (Fig 1D), with an associated mega cisterna magna. The remainder of the brain appeared normal. Chromosome analysis was grossly normal but was sent for specific analysis of Xq28.
Discussion
Neurons that form the cerebral cortex are generated in the telencephalic germinal matrix, which is a vascular, densely cellular zone within the walls of the telencephalic vesicles. Neuroblasts proliferate within the germinal matrix during the 7th and 8th weeks of gestation; during the 8th week, they start to migrate from the subependymal region toward the pial surface (18). This process of migration lasts for approximately 2 months, until the 25th or 26th gestational week, and is maximal between 8 and 16 weeks. It seems very likely that some cases of periventricular heterotopia represent failed migration (5), but some may result from an abnormal proliferation of neuroblasts in the periventricular region to form disorganized gray matter nodules (2) or from failure of regression or apoptosis of neuroblasts within the germinal matrix.
Subependymal heterotopia are frequently associated with epilepsy; they may be isolated or may occur in conjunction with other cerebral malformations such as callosal agenesis, Chiari II malformations, polymicrogyria, and basilar cephaloceles (19, 20). In 1994, Huttenlocher et al (21) presented the reports of a large family in which subependymal heterotopia were seen exclusively in female members, with a high incidence of spontaneous abortion, suggesting an X-linked inheritance pattern. It was subsequently determined that this family and others with bilateral subependymal heterotopia have mutations within the distal long arm of the X chromosome (Xq28) (2). The specific gene product was recently identified as a protein that has been named filamin-1. This protein functions in the cross-linking of intracellular actin, essential for cell migration, and also has important functions in the vascular and immune systems (5). Because subependymal heterotopia is an X-linked condition, the phenotype differs between male and female persons. Affected female persons usually have normal or mildly impaired intellect, with relatively late onset of various epilepsy disorders (2, 3, 21, 22). Fox et al (5), in 1998, found several associated non-neural abnormalities in the two families they examined. In female members, these included abnormalities of major blood vessels such as patent ductus arteriosus, bicuspid aortic valve, and stroke at an early age (5). In male persons, who have only one X chromosome, the condition is often fatal in early embryonic life (2, 21), possibly because filamen 1 has essential functions in hemostasis and in blood vessel development (5). Affected male persons who survive the embryonic and fetal periods have more severe neurologic and intellectual disabilities than do affected female persons (23). Commonly associated cerebral malformations found in both male and female persons included callosal agenesis and cerebellar hypoplasia or other subtle cerebellar malformations (5, 23). The early diagnosis of affected fetuses in families with this condition may allow appropriate planning for delivery and management and genetic counseling for future pregnancies.
Sonography is a well-tested, readily available, safe method for the diagnosis of many fetal CNS abnormalities (24). MR imaging, however, offers superior soft-tissue contrast, with no interference from fat or surrounding bony structures, and is a promising technique in situations in which the sonographic diagnosis is not certain (6−16). Although fetal MR imaging has been investigated for more than 15 years (16), difficulties with fetal movement during early gestation, compounded by maternal discomfort and movement, have limited its use. The development of faster sequences and stronger gradients has improved the quality of images that can be obtained in short imaging times (13), but in early gestation, the fetus is highly mobile within a relatively large liquor volume, and movement remains a considerable problem. Both T1- and T2-weighted images are required for optimal tissue characterization. Commercially available T2-weighted half-Fourier single-shot fast spin-echo sequences can be performed in a few seconds, allowing multiple images to be acquired in a single maternal breath hold. Unfortunately, most standard T1-weighted sequences that are currently commercially available require longer imaging times to achieve adequate resolution during a single breath hold. It can be difficult to obtain images quickly in orthogonal planes, particularly if there is fetal movement between images. A volume acquisition is potentially useful for this reason but unfortunately takes longer to acquire, and motion becomes a major problem. Although MR techniques have not evolved to the point at which they can be used for routine screening, certain families with positive histories of neurologic disease may require a more accurate diagnosis; in such situations, MR imaging may be indicated early in gestation. Fetal immobilization may be achieved by maternal sedation with benzodiazepine (15) or by injection of pancuronium (14) into the umbilical vein. Both techniques have been used successfully, but both have significant associated risks to the fetus, and we do not currently use these techniques.
Gray matter heterotopia are readily diagnosed postnatally by MR imaging and may also be identified on cranial sonograms and CT scans. Neonatal sonography shows irregular ventricular margins and a hyperechoic periventricular band or nodules that are more echogenic than white matter but less echoic than the choroid plexus (25). Heterotopia are difficult to differentiate from hamartomas of tuberous sclerosis, and CT or MR imaging is usually required to clarify the diagnosis. On CT scans and MR images, subependymal heterotopia appear as nonenhancing periventricular nodules that are isointense to gray matter on all pulse sequences. They may be diffuse and symmetric, forming a contiguous undulating bandlike mass around the lateral ventricles, or may be nodular and noncontiguous. Associated cortical malformations are unusual. There have been reports of the antenatal diagnosis of subependymal heterotopia in the third trimester (33 weeks) (12) or in association with agenesis of the corpus callosum (6) or other callosal malformations (10), which alerted the examiners to the possibility of concurrent pathologic abnormality. In our case, a slightly nodular ventricular wall (Fig 1A–B) with a mega cisterna magna raised the suspicion of heterotopia, even at the early gestational age of 23 weeks. The subsequent T2-weighted MR images showed nodules with signal intensity similar to that of gray matter protruding into the ventricular lumen (Fig 1C). Isointensity with gray matter could not be confirmed on T1-weighted images, because motion and poor spatial resolution degraded the sequences.
The appearance of the fetal MR image is not unequivocal; the differential diagnosis includes tuberous sclerosis and subependymal hemorrhage. The subependymal hamartomas of tuberous sclerosis are hyperintense on T1-weighted images and hypointense on T2-weighted images in neonates and infants (26−28). These hamartomas can also be detected by imaging in the antenatal period. In a study conducted by Sonigo et al (11) in 1996 of eight fetuses with cardiac abnormalities, MR imaging showed hyperintense periventricular nodules on T1-weighted images of five fetuses subsequently confirmed to have tuberous sclerosis. The antenatal appearance of heterotopia on T1-weighted images is likely to be very similar to this, although the suboptimal quality of our T1-weighted images did not allow us to confirm hyperintensity. The coexistence of other typical associated lesions (mega cisterna magna or callosal agenesis in heterotopia, cortical tubers or cardiac rhabdomyomas in tuberous sclerosis) may be helpful in making this differentiation. Hemorrhage occurring in the germinal matrix may cause small periventricular lesions. The frequency of germinal matrix hemorrhage in utero has not been well studied; however, the signal characteristics of hemorrhage at certain stages could closely mimic subependymal heterotopia, with relative hyperintensity on T1-weighted images and hypointensity on T2-weighted images. Other factors such as the presence of intraventricular hemorrhage, hydrocephalus, and rapid evolution may indicate hemorrhage rather than heterotopia. Generally, subependymal hemorrhages have a typical sonographic appearance and evolution, and the combination of sonography and MR imaging will likely show the difference between hemorrhage and heterotopia in most cases.
The normal presence of the periventricular germinal matrix is a confounding feature in the detection of subependymal heterotopia. It is not known whether the typical antenatal appearance of sub-ependymal heterotopia differs from the appearance of the normal germinal matrix up to 26 weeks. The MR appearance of the germinal matrix is that of gray matter (7, 12). Therefore, an understanding of the development of the germinal matrix is necessary to recognize its normal appearances at various stages of development on fetal MR images. This understanding will help to differentiate the normal germinal matrix from the periventricular heterotopia. From 13 weeks' to approximately 24 to 26 weeks' gestation, the germinal matrix increases in volume because of proliferation of neuroblasts and glia. After 26 to 28 weeks, it decreases in size, rapidly at first and then more gradually (17). The matrix surrounds the entire ventricular wall until approximately 30 weeks' gestation, and it is thicker around the ventrolateral wall, roof, and medial wall of the lateral ventricles than over the corpus striatum. As it loses volume, the matrix loses continuity first around the third ventricle; it then progressively regresses around the occipital horns and trigones and subsequently around the temporal horns. From 36 weeks' gestation to term, normally only scattered matrix cells are present in the subependymal region, except for a thick layer between the caudate nucleus and thalamus (known as the ganglionic eminence). This fragments during the first 3 months after birth, and the periventricular matrix has normally completely disappeared by the end of the first postnatal year (1).
Several investigators have examined the normal MR appearance of the germinal matrix during its development and regression. Girard et al (7), in 1995, performed an in vivo study of 33 normal fetuses ranging from 21 to 38 weeks' gestation. Between 23 and 28 weeks' gestation, they found three layers of hyperintense bands within the cerebral hemispheres on T1-weighted images, corresponding, from central to peripheral, to the germinal matrix, the migrating neuroblasts, and the developing cortex. After 28 weeks' gestation, the brain had a more homogeneous appearance on T1-weighted images and the germinal matrix became thinner (7). Chong et al (29), in 1996, examined 28 fetal brain specimens with an age range from 9 to 24 weeks (29). Using a spoiled gradient-recalled sequence to optimize differentiation between gray and white matter, the germinal matrix was seen as a band of increased signal along the lateral margins of the lateral ventricles after 13 weeks' gestation. The discrepancy between the first appearance of the germinal matrix on MR images (at 13 weeks) and the onset of cellular proliferation in this region (at 7 to 8 weeks) (17, 18) was thought to reflect the limited resolution of MR imaging. The germinal matrix became thinner from 19 to 21 weeks' gestation, somewhat earlier than was previously described (7, 17). Brisse et al (30), in 1997, examined the MR images of five fetal brain specimens and obtained histologic correlation for all specimens (30). The germinal matrix was visible on the image of the 27-week specimen but was not seen at 34 weeks, and histologic correlation at this stage showed no residual matrix. The timing of regression of the germinal matrix in these postmortem studies differed from that noted by Battin et al (31) and Evans et al (32) in MR studies of live premature infants. Those studies showed that the germinal matrix had an appearance similar to that previously reported but noted that it may be visible after 32 weeks and, in some cases, could be seen at term (31, 32). These studies and others have not established whether the temporal evolution of the germinal matrix is similar in fetuses compared with prematurely born neonates of similar postconceptional ages or whether the in vitro studies truly reflect the in vivo events.
The images acquired in this case suggest that subependymal heterotopia can be detected by sonography and MR imaging well before any regression of the germinal matrix occurs. Nodules were visible at a stage when the germinal matrix would be expected to be close to its maximum volume. The heterotopic nodules were isointense to cortex on T2-weighted MR images and were of low signal intensity compared with the adjacent germinal matrix. The relatively short T1 and T2 relaxation times of the germinal matrix are thought arise from its dense cellularity (7, 30) or an alteration in free water content (33); however, it is hard to understand why heterotopic nodules should have a different signal than germinal matrix, considering that the cellular composition of both is similar (2). The irregularity of the ventricular wall is certainly a useful clue to the presence of heterotopia in that we expect the ventricular walls to remain smooth as the germinal matrix undergoes its normal involution. Heterotopia may therefore be more visible in those regions in which the germinal matrix regresses first, with the thinness of the germinal zone highlighting the irregularity of the wall and the contrast differences between heterotopic gray matter and the germinal matrix. This would probably be most noticeable around the lateral ventricular margins, as in the present case. After 30 weeks, the germinal matrix has virtually completely regressed, and by this stage, periventricular heterotopia would be expected to be easily visible.
How early, then, can subependymal heterotopia be diagnosed by imaging? In theory, the heterotopia should be visible as nodules of thickened germinal zone in the early second trimester, as soon as the lateral ventricles are easily seen by sonography, and the neuroblasts have begun their migration to the cortex. Once the neuroblasts have left the germinal matrix, it may not matter that the germinal matrix cannot be resolved by sonography, because the major visible abnormality is irregular ventricular walls (Fig 1A–B). Similarly, on an early MR image, irregularity of the ventricular wall may be the most easily identifiable abnormality, and this may be appreciated best on T2-weighted sequences. Nevertheless, the tiny size of the brain (and hence the periventricular nodules) and the degree of fetal movement at this very early stage would render the diagnosis difficult on the basis of MR images and sonograms.
In conclusion, subependymal heterotopia are detectable in the fetus by both sonography and MR imaging as early as 23 postconceptional weeks. MR imaging is a valuable adjunct to sonography in the mid second trimester evaluation of fetuses with a family history of X-linked subependymal heterotopia or when sonographic findings raise the possibility of underlying heterotopia.
Footnotes
↵1 Address reprint requests to A. James Barkovich, MD, Department of Radiology, Section of Neuroradiology, L 371, University of California, San Francisco, 505 Parnassus Avenue, San Francisco, CA 94143.
References
- Received August 16, 1999.
- Copyright © American Society of Neuroradiology