Abstract
BACKGROUND AND PURPOSE: Children with dyslexia have difficulty learning to recognize written words owing to subtle deficits in oral language related to processing sounds and accessing words automatically. The purpose of this study was to compare regional changes in brain lactate between dyslexic children and control subjects during oral language activation.
METHODS: Brain lactate metabolism was measured during four different cognitive tasks (three language tasks and one nonlanguage task) in six dyslexic boys and in seven control subjects (age- and IQ-matched right-handed boys who are good readers) using a fast MR spectroscopic imaging technique called proton echo-planar spectroscopic imaging (1-cm3 voxel resolution). The area under the N-acetylaspartate (NAA) and lactate peaks was measured to calculate the lactate/NAA ratio in each voxel.
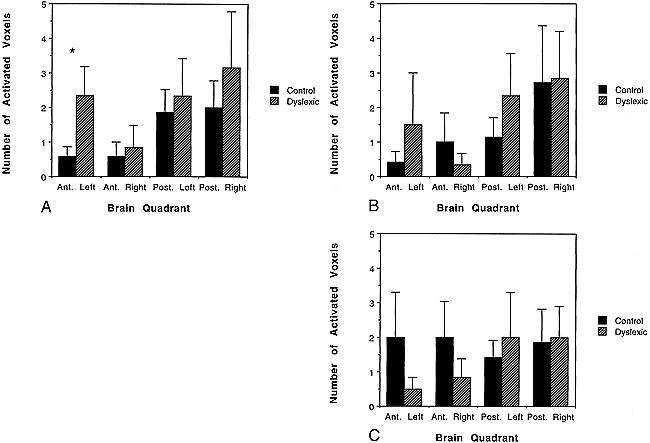
RESULTS: Dyslexic boys showed a greater area of brain lactate elevation (2.33 ± SE 0.843 voxels) as compared with the control group (0.57 ± SE 0.30 voxels) during a phonological task in the left anterior quadrant. No significant differences were observed in the nonlanguage tasks.
CONCLUSION: Dyslexic and control children differ in brain lactate metabolism when performing language tasks, but do not differ in nonlanguage auditory tasks.
Dyslexia, or specific reading disability, is the most frequently occurring learning disability and the most common disorder of childhood. Estimated to affect 5% to 15% of children, dyslexia is characterized as unexpected underachievement in reading for one's intellectual ability. It has been well-established that it is a language-based disorder, often caused by deficits in phonological processing (1–4). Although dyslexia is a genetic disorder (5), with reported linkage to chromosomes 6 and 15 (6, 7), its phenotypic expression (V. Berninger, et al, unpublished data, 1999) depends on the environment as well as heredity (8, 9). Twenty years of behavioral evidence support two major causal mechanisms in dyslexia: deficient phonological processing of spoken words (1–4) and inability to visually access names in the lexicon (mental dictionary) automatically (10, 11). The consequence of these deficiencies is poor reading, which differs from good reading in verbal efficiency (4). Several investigators have found associations between neurophysiological abnormalities and dyslexia (12–16). Functional neuroimaging studies with 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) indicate that adult dyslexics have focal increases in glucose metabolism (12, 13), suggesting either inefficient processing or the activation of compensatory pathways (14).
Based on what is known about the metabolic role of lactate and glucose during brain activation, we hypothesized that brain functional inefficiencies would exist in dyslexics, specifically manifested as elevated lactate covering a greater area of the brain during language. Lactate is known to be metabolized in the brain as a neuronal substrate (17) and also as a by-product for glucose metabolism during brain activation. MR spectroscopy has previously been used to demonstrate lactate activation (increase in lactate) in healthy adults during visual, auditory, and cognitive tasks (18–22). In these studies, lactate was observed to increase rapidly during sensory stimulation in a regionally specific manner.
We used a novel noninvasive technique called proton echo-planar spectroscopic imaging (PEPSI) (23) to investigate metabolic brain activation during oral language tasks in dyslexic and control children. Previous neuroimaging studies of language have used either PET (24) or functional MR imaging (25), which uses blood oxygenation level–dependent image contrast changes to map out regional brain activation (an indirect measure of metabolism). Functional MR spectroscopy performed with the PEPSI technique is an alternative approach for detecting regional brain activation and measuring tissue-based lactate changes (a direct measure of metabolism) produced by a temporary mismatch of oxygen delivery and consumption in response to neuronal activation (26). For this study, we specifically tested the hypothesis that a greater area of brain activation (higher voxel counts) would occur with language processing in dyslexic children than in control subjects.
Methods
Study Design
Six dyslexic and seven nondyslexic (control) boys were imaged using the PEPSI technique (23) while they performed four different cognitive tasks. The dyslexic and control groups were well-matched in age, IQ, and head size (number of total voxels) but not in reading skills, in which they demonstrated marked differences, as described below. The experimental tasks were designed to activate phonological and lexical access functions of the brain while a tone task was used to activate auditory nonlanguage functions of the brain. Scanner noise and passive listening (to word lists used in both the phonological and lexical access tasks) were two control tasks used to subtract out low-level acoustic and nonspecific stimulus effects, respectively. The phonological and lexical access tasks engage additional linguistic processes beyond those required for passive listening of language. The unique brain activation evoked by processing rhymes or accessing word meanings, independent of brain activation due to the characteristics of word stimuli, was assessed by subtracting out the passive listening condition from the phonological and lexical access tasks. The component of brain activation related to processing requirements for auditory functions not specific to language was assessed by subtracting out the scanner noise from the tone judgments.
MR Imaging and Spectroscopy
Conventional MR imaging and PEPSI were performed on a clinical 1.5-T Signa MR imaging system from General Electric equipped with version 5.4 software and a custom-built radiofrequency coil developed by Hayes et al (27). MR images were acquired in the sagittal plane (600/20 [TR/TE]) and also in the axial plane (2000/35,80). The custom-designed coil was necessary to acquire MR spectroscopic data with high enough signal-to-noise ratio to detect the small lactate peak. The coordinates of the sylvian fissure and surrounding language-related structures were determined from the sagittal and axial images and used to determine the axial section for spectroscopic imaging. The areas sampled with PEPSI were based on the work of Ojemann et al (28), which invasively demonstrated language activation in the anatomic region encompassing the sylvian fissure and adjacent opercula. Deeper subcortical structures were also included that are associated (through neuronal connectivity) with the cortical areas. Proton spectra were acquired using PEPSI, a spin-echo pulse sequence developed by Posse et al (23) that allows fast spectroscopic imaging that is 32 times faster than conventional hydrogen spectroscopic imaging for the same spatial resolution. Parameters for data acquisition were as follows: 4000/272/2, 32 × 16 spatial matrix, 512 echoes in the echo-planar acquisition, 32 complex points per echo, full echo acquisition, 24-cm field of view, and 20-mm section thickness. Spatial resolution was approximately 1 cm3. Data were processed as described previously (22). The metabolites were integrated using the following procedure: 1) magnetic field inhomogeneity (B0) shifts were corrected by finding the maximum point of the NAA peak and resetting the ppm scale to 2.0 ppm for each spectrum; 2) the average baseline was determined from 32 points to the right of 0.0 ppm; 3) the maximum intensity point of the peak was determined within a set spectral range (NAA = 2.0 ± 0.07, lactate = 1.3 ± 0.1 ppm); and 4) integration was performed by summing the spectral intensities for the NAA and lactate for the ppm ranges specified in step 3.
Subject Characterization
The University of Washington Human Subjects Institutional Review Board approved this study, and each subject (as well as parent/guardian) gave written, informed consent. All subjects were right handed (90% to 100% on the Edinburgh handedness scale) (29). The control subjects had a history of learning to read easily and were reading above normal for age (average was 1 SD above mean for age using the Woodcock Reading Mastery Test-Revised [WRMT-R]) (30). The dyslexic boys had a developmental history of extreme difficulty in learning to read despite many forms of extra assistance at school, and also had a family history of multigenerational dyslexia, which was confirmed in a concurrent family genetics study (W. Raskind, personal communication) at our center. The dyslexic boys were reading on average 1.66 SD below the mean for age using the WRMT-R test (30). In addition, all the dyslexic boys were shown to have a triple deficit in three skills that predict ease of learning to read and response to intervention, phonological (phoneme segmentation and/or memory for spoken nonwords), rapid automatized naming, and orthographic (speed of coding written words and/or accuracy of representing them in memory) (31). Based on independent t-tests, the seven control subjects (mean = 127.3, SD = 10.8) and six dyslexic boys (mean = 124.3, SD = 11.1) did not differ in age in months: t(11) = 0.49, P = .637. Likewise, the control subjects (mean = 15.6, SD = 3.2) and dyslexic children (mean = 13.2, SD = 1.6) did not differ in age-corrected WISC-III vocabulary scores (t[11] = 1.68, P = .12), which provide the best estimate of full-scale IQ. However, the control and dyslexic boys did differ significantly in age-corrected standard scores for reading real words on the word identification (WI) subtest of the WRMT-R test and for reading pseudowords on the Word Attack (WA) subtest of the WRMT-R (t[11] = 6.81, P < .001 on the WI subtest and t[10] = 6.02, P < .001 on the WA subtest). The differences for both real-word reading (WI, control subjects: mean = 115.1, SD = 9.2; dyslexics: mean = 75.5, SD = 11.8) and pseudoword reading (WA, control subjects: mean = 110.2, SD = 6.8; dyslexics: mean = 79.0, SD = 10.7) were large as well as statistically significant.
Language Tasks
During MR imaging, the children were asked to listen to aurally presented words, nonwords, and tone pairs at a rate of one stimulus pair every 4 seconds. Language stimuli were composed of four groupings of word pairs, crossed for lexical status (word vs nonword) and sound similarity (rhyming vs nonrhyming), resulting in four sets of stimuli: word/word:nonrhyming (eg, FLY-CHURCH); word/word:rhyming (FLY-EYE), word/nonword:nonrhyming (CROW-TREEL); word/nonword:rhyming (MEAL-TREEL). Nonwords, such as TREEL, allow assessment of sound processing without any meaning cues. The presenting order of word pair-types were counterbalanced and thus the ordering effects were controlled for. During rhyming (phonological task), subjects listened to the same stimulus material and judged whether the word pairs rhymed or did not rhyme; whether words were real was irrelevant. During the lexical access condition, subjects listened to the word pairs and judged whether the word pairs contained two real words or contained a nonword; whether the words rhymed was irrelevant. Thus, the same stimulus lists were used for lexical access and rhyming, only the task instructions changed. Subjects indicated their rhyme and lexical decisions by raising cards held in the right and left hands (the hand used to signal a Yes response was counterbalanced across subjects). During passive listening, subjects listened to the same stimuli but were instructed to alternately raise the left and right hand without making any judgments on the stimuli. For the tone judgment task, five pure tones (329.6 Hz, 350.0 Hz, 415.3 Hz, 440.0 Hz, and 523.0 Hz) were grouped into pairs of identical tones or different tones. Subjects were asked to raise one hand if the tones were identical and to raise the opposite hand if the tones were different. The subjects were tested for accuracy of their responses for all tasks during a prescan training session and during the actual MR examination. For the tone subtraction, the baseline scanner noise was used instead of passive listening. A recovery period of 5 minutes between tasks was based in part on the lactate recovery measurements by Frahm et al (26).
Data Analysis
To affirmatively evaluate focal brain activation, z-score maps were created from the lactate/NAA ratios based on the following equation: 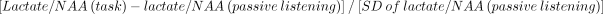 where (task) refers to the task given during the MR examination: phonological, lexical access, or tone differentiation. The SD of the lactate/NAA was calculated for each subject using all valid spectra of the control task (either passive listening or scanner noise). This z-score was calculated for all voxels that contained valid spectra for each language condition (22). Definition of lactate elevation was based on z-scores greater than 2.0 on a voxel-by-voxel basis. The number of voxels with elevated lactate within each quadrant was counted for each subject.
where (task) refers to the task given during the MR examination: phonological, lexical access, or tone differentiation. The SD of the lactate/NAA was calculated for each subject using all valid spectra of the control task (either passive listening or scanner noise). This z-score was calculated for all voxels that contained valid spectra for each language condition (22). Definition of lactate elevation was based on z-scores greater than 2.0 on a voxel-by-voxel basis. The number of voxels with elevated lactate within each quadrant was counted for each subject.
The PEPSI data were analyzed to ascertain the number of voxels (computerized volume elements) with elevated lactate in four regions of the brain. Because regional specificity of lactate response is not well established and also because of the large variability among subjects in the spatial location of the lactate response, we divided the spectroscopic imaging section into four quadrants. The brain was divided into four quadrants based on left to right (brain midline defined on the axial MR image) and anterior to posterior (using the midpoint of the thalamus as a landmark). Inferential statistics were used to compare relative activation for each group in each brain quadrant on each task. ANOVA was used to test for differences in the number of activated voxels between control and dyslexic subjects. The number of valid voxels for the dyslexic group was not significantly different from that of the control group (controls: mean = 160.6, SD = 6.9; dyslexics: mean = 166, SD = 19; t[11] = 0.76, P < .48).
The lactate/NAA ratio was used to normalize for radiofrequency inhomogeneity, variable CSF contribution, and also to standardize the lactate signal across subjects. An automated computer-software mask was applied to the spectra to ensure that the MR lactate signal was not contaminated with scalp lipid signal (22). A true lactate peak was differentiated from lipid contamination on the basis of MR frequency, which was determined from an in vitro lactate measurement (the lipid peak MR frequency was not the same as lactate frequency). Additionally, in previous work, we varied the TE to assess J-coupling properties of the peak at 1.3 ppm, which identified this peak as lactate (32).
Results
Dyslexic boys had significantly more brain voxels with elevated MR lactate levels (2.33 ± SE 0.843) than did the control group (0.57 ± SE 0.30) during a phonological task in the left anterior quadrant (ANOVA: F[1,11] = 4.41, P = .05, see Figs 1 and 2). None of the other quadrants were significantly different between dyslexic and control subjects for the other three quadrants during the phonological task (P values ranged from .62 to .74), during the lexical access task (P values ranged from .37 to .96), during the tone task (P values ranged from .32 to .92), or during the scanner noise alone (P values ranged from .23 to .99). The fact that there was no difference between the dyslexic and control subjects during the tone task implies that the difference between dyslexics and control subjects is specific to auditory language function and not to nonlinguistic auditory function. Behavioral data during scan sessions demonstrated that all subjects performed the tasks above chance levels, showing attentiveness to the task, and that the dyslexics had a significantly lower correct response rate in responding to phonological tasks than did the control group (dyslexics: mean = 89.6, SD = 3.9; controls: mean = 96.7, SD = 2.9; t[10] = 3.78, P < .004). Behavioral differences between the dyslexics and control subjects during the tone task, however, were not statistically significant (t[10] = 1.7, P = .12).
Bar graphs show number of activated voxels (as defined by MR spectroscopic lactate increases) in the four quadrants of the brain for both dyslexic and control children.
A–C, Graphs of phonological task data (A), lexical access task (B), and tone task (C). (Error bars are standard error of the mean; asterisk indicates dyslexic versus control comparisons that were significantly different).
Discussion
Our main finding was that the dyslexic children in comparison with nonimpaired children had a larger regional distribution of metabolic activation, characterized by the number of voxels with elevated lactate, in the left anterior quadrant during the phonological task. This greater regional activation cannot be directly attributed to the dyslexic children's reading difficulty, because the tasks were auditory and did not involve reading per se. Concurrently collected behavioral data verified that the subjects were attentively listening to the auditory stimuli and also that the dyslexic children were less accurate than the control group on the phonological task. Our findings suggest that the dyslexic brain has a larger regional distribution of metabolic activation (as evidenced by the increased number of voxels with elevated lactate) than normal brain during the mental process of accomplishing the same linguistic task (although the dyslexic children made more linguistic errors). This metabolic abnormality points toward a phonological processing problem located in the left anterior quadrant (this quadrant includes the left frontal lobe and language areas surrounding the sylvian fissure). This increased metabolic energy demand may be the neurologic substrate underlying the behavioral phenomenon described by the verbal efficiency theory (4). According to this theory, poor readers must exert more mental effort (because of inefficient language processing) than good readers to accomplish the same task. Further work will be required to determine whether the greater activation reflects inefficient processing within language areas or the activation of compensatory pathways (ie, a functional brain pathway outside the normal brain activation region that is established to compensate for dyslexic deficits).
Increases in the MR lactate signal have been observed in the occipital cortex during photic stimulation (19, 20) in the auditory cortex during 1-kHz tone pulses (33), in the basal ganglia during finger movements (34), and in the limbic region of the brain during seizure activity (35). The increase in lactate is thought to be related to an increase in glucose metabolism during neuronal stimulation.
Recent studies using functional MR imaging by Shaywitz et al (15) have also shown that adult dyslexic subjects exhibit different brain activation patterns than nonimpaired subjects during a phonological language task as compared with an orthographic coding task. These investigators found significant overactivation in the left inferior frontal gyrus (see Fig 2 in [15]) in the dyslexic subjects as compared with control subjects (15). These findings for adults are consistent (although more anatomically localized) with our findings for children, inasmuch as we also found regional metabolic overactivation relative to control subjects in the left anterior quadrant, which included the left inferior frontal gyrus. Using FDG-PET, Hagman et al (12) found higher metabolism in the medial temporal lobe bilaterally in adult dyslexics during an auditory syllable discrimination task. FDG and lactate are both involved in carbohydrate metabolism of the brain and both are involved in energy metabolism during neuronal activation. Rumsey et al (36), using 15O PET to measure blood flow, found that the left inferior frontal cortex is involved in phonological processing. One difference between our results and those in the studies mentioned above is that the pattern of metabolic activation that we observed with lactate included subcortical areas as well as cortical areas (cortical activation areas are reported by the PET and functional MR imaging studies). The subcortical activation areas that we observed may reflect neuronal connectivity from the adjacent cortical areas involved with language.
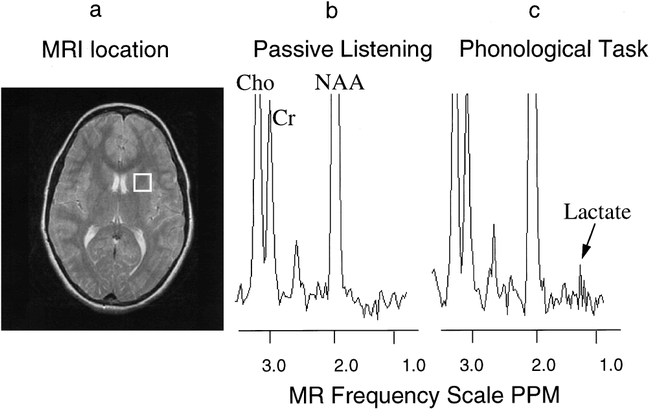
MR image and proton spectra from an activated brain region of one dyslexic subject.
A, MR image with white box indicating the brain region measured with MR spectroscopy.
B, Proton MR spectrum from the white box brain region during the passive listening task.
C, Proton MR spectrum from the white box brain region during the phonological task. The intensity axis of the spectra is scaled so that the lactate can be seen easier; however, choline (Cho) and NAA are scaled off the figure. Note the increase in the lactate peaks during the phonological task as compared with passive listening.
Our findings are important because they shed new light on brain mechanisms involved with dyslexia at a developmental stage when dyslexia is amenable to treatment (8, 37). Because most children respond positively to instructional interventions, many educators have rejected the notion that reading problems are brain-based. Functional brain differences between dyslexics and control subjects such as those demonstrated by this study add evidence that dyslexia is a brain-based disorder. We have shown that there is a difference in the regional distribution of lactate elevation between dyslexics and control subjects; however, it is premature to use this technique as a diagnostic tool for dyslexia.
Conclusion
We have established differences in the regional distribution of brain lactate metabolism between dyslexic and control children (matched for age, IQ, and head size) during an aurally presented phonological task. Our study extends previous work that found differences in patterns of cerebral blood flow between adult dyslexics and control subjects in visually presented functional MR imaging reading tasks (15) and in phonological and orthographic tasks during PET scanning (17).
Acknowledgments
We gratefully acknowledge contributions from Darrell Hochman and Martin Kuschmerick to this manuscript, and editorial assistance from Jane Johnson.
Footnotes
↵3 Supported by a Special Multidisciplinary Learning Disabilities Center grant from NIH (NICHD), P50 HD33812.
Presented in part at the annual meeting of the Cognitive Neuroscience Society, San Francisco, CA, April 1998.
Address reprint requests to Todd L. Richards, PhD, Department of Radiology, Box 357115, University of Washington, Seattle, WA 98195.
References
- Received September 29, 1998.
- Accepted after revision March 24, 1999.
- Copyright © American Society of Neuroradiology