Abstract
BACKGROUND AND PURPOSE: MR imaging, PET, and ictal SPECT have been studied extensively as individual techniques in the localization of epileptogenic foci, but only a few comparative studies have been done. We evaluated the concordance rates of ictal video/EEG, MR imaging, PET, and ictal SPECT to compare the sensitivities of these imaging methods in the lateralization of epileptogenic foci.
METHODS: The study included 118 consecutive patients who underwent surgery for medically intractable epilepsy and who were followed up for 12 months or more. MR imaging was compared retrospectively with ictal video/EEG, FDG-PET, ictal 99mTc-HMPAO SPECT, and invasive EEG as to their ability to localize the epileptogenic focus; the pathologic findings served as the standard of reference.
RESULTS: MR imaging was concordant with video/EEG, PET, and ictal SPECT in 58%, 68%, and 58% of patients, respectively. With the pathologic diagnosis as the standard of reference, MR imaging, PET, and ictal SPECT correctly lateralized the lesion in 72%, 85%, and 73% of patients, respectively. Of the patients with good outcomes, MR imaging, PET, and ictal SPECT were correct in 77%, 86%, and 78%, respectively. In the good outcome group, MR imaging was concordant with PET and ictal SPECT in 73% and 62% of patients, respectively. Of 45 patients who underwent invasive EEG, MR imaging was concordant with the invasive study in 47%; PET in 58%; and ictal SPECT in 56%. Of 26 patients with normal MR findings, PET and ictal SPECT correctly lateralized the lesion in 80% and 55%, respectively.
CONCLUSION: Overall concordance among the techniques is approximately two thirds or less in lateralizing epileptogenic foci. PET is the most sensitive, even though it provides a broad approximate nature of the epileptogenic zone, which is not adequate for precise surgical localization of epilepsy. PET and/or ictal SPECT may be used as complementary tools in cases of inconclusive lateralization with ictal video/EEG and MR imaging.
Epilepsy is a common neurologic disorder, affecting at least 0.5% of the general population (1). Despite medical therapy, some patients with epilepsy have persistent seizures and may benefit from surgery. Surgical therapy in patients with medically intractable epilepsy requires accurate, noninvasive evaluation of the epileptic focus (2). Sleep-deprivation electroencephalography (EEG) and video-surface EEG monitoring are the primary laboratory methods of searching for localizing ictal activity, but the success rate of EEG methods ranges from 60% to 90% (3). MR imaging, single-photon emission computed tomography (SPECT), and positron emission tomography (PET) have been studied extensively in the evaluation of localizing the epileptogenic focus, but they have mostly been reported as individual imaging techniques. Only a few studies comparing these methods have been published (4, 5), presumably because of the limited availability of the techniques and the continuing advances in technology. The studies that have been done primarily compared their effectiveness with EEG as the standard by which epilepsy was diagnosed. To verify accuracy and to determine the yield of neuroimaging methods for localization of epileptogenic focus, three standards might be used: EEG, pathologic diagnosis of the resected tissue, and outcome after epilepsy surgery. EEG localization remains a viable standard for analysis but suffers from differing methodologies with inherently variable accuracy.
The purpose of this study was to assess the concordance rates of ictal video/EEG, MR imaging, PET, and ictal SPECT in lateralization of epileptogenic focus and to compare the sensitivities of these imaging techniques on the basis of pathologic diagnosis and surgical outcome.
Methods
Patient Population
One hundred eighteen patients who underwent surgery for medically intractable epilepsy during the period from October 1994 through September 1996 were included in the study. Seventy-four patients were male and 44 were female; ages at the time of surgery ranged from 8 to 55 years (mean, 27 years). The postoperative follow-up period ranged from 12 to 35 months (mean, 24 months). Preoperatively, it was our intention that all the patients were to have MR imaging, video/EEG monitoring with scalp electrodes, interictal and ictal SPECT, interictal PET, and neuropsychological studies as a routine protocol. In fact, all patients had MR imaging, video/EEG monitoring, and neuropsychological studies; however, owing to equipment problems, unavailable radioactive tracers, inability to inject radiopharmaceuticals for ictal SPECT, or the high cost of PET, only 110 patients had interictal SPECT, 95 had PET, and 77 had ictal SPECT. Forty-five patients, who had either normal MR imaging findings or nonconcordant findings among the noninvasive studies, underwent invasive intracranial EEG studies (mostly with subdural electrodes). There were no aborted studies. Video/EEG monitoring was performed in a special epilepsy ward equipped with a 24-hour video monitoring system in which the recording of ictal semiology by video camera and ictal EEG changes by digital EEG occurred at the same time. Whenever a patient had a seizure episode, the VCR recorded the patient's behavioral changes and the ictal EEG recording started.
MR Imaging
Standard MR imaging was performed on a 1.0-T or a 1.5-T unit with conventional spin-echo T1-weighted sagittal and T2-weighted axial and coronal sequences. Section thickness and gap were 5 mm and 1 mm, respectively. For patients with temporal lobe epilepsy, T2-weighted fast spin-echo sequences with 3-mm-thick sections and T1-weighted 3D magnetization prepared rapid acquisition with gradient-echo sequences with 1.5-mm-thick sections were obtained in the oblique coronal plane of the temporal lobes. The angle of oblique coronal imaging was perpendicular to the long axis of the hippocampus, resulting in a slight variation of angulation from patient to patient. Spatial resolution was approximately 1.0 × 1.0 mm (matrix, 256 × 256 mm; field of view, 25 cm).
PET
Axial raw data were obtained on a PET scanner 60 minutes after intravenous injection of 18F-fluorodeoxyglucose (FDG) (370 MBq) during the interictal period. Acquisition time was approximately 20 minutes. Axial images were reconstructed with a Shepp-Logan filter (cutoff frequency, 0.35 cycles per pixel) and realigned in coronal and sagittal planes. Spatial resolution was 6.1 × 6.1 × 4.3 mm.
SPECT
Baseline axial interictal and ictal SPECT data were acquired using a triple-head camera with a fanbeam collimator after intravenous injection of technetium–99m hexamethylpropyleneamine oxime (99mTc-HMPAO) (925 MBq). Axial images were reconstructed with a Metz filter (× = 1.7–2.0). For ictal SPECT, intravenous injection of the radiotracer was performed during the ictal period, mostly within 30 seconds after seizure onset. The ictal SPECT images were obtained 1 to 3 hours after the seizure activity ceased. Axial images were realigned in coronal and sagittal planes. Section thickness was 5 mm and spatial resolution was 12 × 12 mm.
Surgery and Pathologic Examination
Surgical treatment included anterior temporal lobectomy (n = 81), neocortical resection (n = 31), lesionectomy (n = 5), and corpuscallosotomy (n = 1). Standard anterior temporal lobectomy was performed in all patients in whom temporal lobe epilepsy was demonstrated by video/EEG monitoring, MR imaging, and/or by other techniques. In patients with extratemporal epilepsy, the surgical site was determined primarily by the location of an abnormality on MR images that was concordant with one seen on video/EEG monitoring. When the MR finding was normal, or the abnormality was not concordant with video/EEG or other imaging observations, the site of neocortical resection was determined on the basis of the results of the invasive study, mostly grid recordings, by consensus of an epileptologist and neurosurgeon.
Histopathologic examination of the surgical specimens revealed hippocampal sclerosis (n = 71), cortical dysplasia and/or microdysgenesis (n = 33), benign tumor (n = 5), vascular malformation (n = 6), cysticercosis (n = 2), and focal cerebromalacia (n = 1).
Follow-up and Outcome Classification
All the patients were regularly followed up for assessment of seizure control and psychosocial outcome for 12 months or more. Postoperative seizure outcome at the latest follow-up was classified according to Engel's four categories (6) by an epileptologist. Class I (seizure-free) indicates an absence of seizure activity since surgery, regardless of medication. Class II indicates rare seizures; that is, a few seizures in a year. Class III indicates worthwhile improvement, meaning at least a 75% improvement in seizure frequency compared with preoperative status. Class IV denotes no worthwhile improvement. The patients' outcomes in terms of this classification system are summarized in Table 1.
Patients' outcome rates according to Engel's classification
Preoperative Interpretation of Images and Final Evaluation
All neuroimaging findings were interpreted before surgery without knowledge of the results of the clinical examination, EEG monitoring, or other neuroimaging studies. The original reports of the MR imaging, PET, and SPECT findings were used as the results without blinded reinterpretation. The diagnostic criteria for all the imaging techniques were based on qualitative visual interpretation. MR images were interpreted by an experienced neuroradiologist. For the PET images, the area of the greatest decrease in uptake of FDG was considered the epileptogenic area on the basis of symmetry. In ictal SPECT studies, the area of hyperperfusion relative to the remaining regions was considered to be the epileptogenic region. When hyperperfusion was seen in multiple areas, the most hyperperfused area was regarded as the epileptogenic region. The results of interictal SPECT were excluded, because, in our experience, they showed too low a sensitivity (approximately less than 50%). Both PET and SPECT scans were interpreted by an experienced nuclear medicine specialist.
The radiologic results were compared with one another and with pathologic findings and surgical outcome, with attention to the lateralizing capability, by two radiologists who determined whether the preoperative interpretations of each imaging technique were lateralizing or nonlateralizing and did or did not match the operative site. They were blinded to the results of each imaging study and to the outcome results.
The term concordant was used when focal or diffuse abnormal findings, as manifested on MR images, PET studies, or ictal SPECT scans, were consistent with one another or when the abnormality was seen in the same area as that on the pathologic examination. When the abnormality on the PET or SPECT study was rather diffuse but the lesion was seen on the same side, not far from that on other imaging studies or on the pathologic examination, the finding was classified as correct lateralization. When the abnormal finding on PET or SPECT scans was seen far remote from that on other imaging studies or on the pathologic examination (ie, right frontal lobe on PET or SPECT images and right occipital lobe on the invasive study), it was classified as nonconcordant. The χ2-test was used for statistical analysis.
Results
Preoperatively, MR imaging was concordant with video/EEG, PET, and ictal SPECT in 58% (69/118), 68% (65/95), and 58% (45/77) of patients, respectively. The difference in the concordance rate (68% versus 58%) was statistically significant (P = .001). PET was concordant with ictal SPECT in 71% (44/62) of patients. MR imaging, PET, and ictal SPECT were concordant in 55% (34/62) of patients (Fig 1). Of the 45 patients who had invasive intracranial EEG studies, MR imaging was concordant with the invasive study in 47% (21/45), PET in 58% (19/33), and ictal SPECT in 56% (20/36). When using the pathologic diagnosis as the standard of reference, MR imaging was correct in 72% (85/118) of patients. PET and ictal SPECT were concordant with the pathologic diagnosis in 85% (81/95) and 73% (56/77) of patients, respectively. The difference in the concordance rate (72% versus 85%) was statistically significant (P = .05). In patients with temporal lobe epilepsy (n = 92), MR imaging was concordant with the pathologic diagnosis in 78% (72/92), PET in 88% (69/78), and ictal SPECT in 77% (44/57); whereas in patients with extratemporal epilepsy (n = 26), MR imaging, PET, and ictal SPECT were concordant with the pathologic diagnosis in 50% (13/26), 71% (12/17), and 60% (12/20), respectively (Table 2).
Concordance rates between imaging studies and pathologic diagnosis
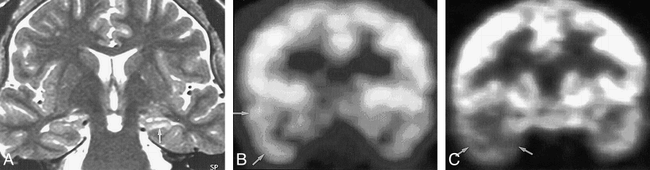
Concordance among MR imaging, ictal SPECT, and PET in a 51-year-old woman with right temporal lobe epilepsy.
A, Oblique coronal fast spin-echo T2-weighted MR image (4000/120/4 [TR/TE/excitations]) shows atrophy and hyperintensity of right hippocampus (arrow).
B, Ictal SPECT scan shows hyperperfusion in right temporal lobe (arrows). Radioactivity ratio between right temporal cortex/left temporal cortex was 2.4:1.
C, FDG-PET scan shows hypometabolism in right temporal lobe (arrows). Radioactivity ratio between right temporal cortex/left temporal cortex was 0.4:1.
After anterior temporal lobectomy, pathologic diagnosis was hippocampal sclerosis. Engel's outcome was class I.
MR imaging findings were apparently normal in 22% (26/118) (14 patients with temporal lobe epilepsy, seven with frontal lobe epilepsy, three with occipital lobe epilepsy, and two with multifocal seizure foci). Among the 26 patients with normal MR imaging findings, PET correctly lateralized the lesion in 80% (16/20), ictal SPECT in 55% (11/20), and invasive EEG in 82% (14/17), as compared with the pathologic diagnosis (Table 3). Of the 95 patients who had both MR imaging and PET, the following abnormalities were apparently missed by MR imaging and correctly depicted by PET: cortical dysplasia (n = 4), microdysgenesis (n = 9), hippocampal sclerosis (n = 2), and focal neuronal loss (n = 1).
Concordance rates with pathologic diagnosis in patients with normal MR imaging findings (n = 26)
When Engel's outcome classification system was used as the standard of reference, among the patients with a good outcome (classes I and II, n = 96), MR imaging was correct in 77% (74/96), whereas PET and ictal SPECT correctly lateralized the lesion in 86% (68/79) and 78% (47/60), respectively. The difference in the concordance rate (77% versus 86%) was statistically significant (P = .004). MR imaging, PET, and ictal SPECT were all concordant in 59% (29/49) of patients (Fig 1). In patients with temporal lobe epilepsy (n = 79), MR imaging correctly lateralized the lesion in 85% (67/79), PET in 90% (61/68), and ictal SPECT in 83% (39/47); whereas in patients with extratemporal epilepsy (n = 17), MR imaging, PET, and ictal SPECT correctly lateralized the lesion in 41% (7/17), 64% (7/11), and 62% (8/13), respectively (Table 4). In the good outcome group (Engel's class I or II, n = 96), MR imaging was concordant with PET and ictal SPECT in 73% (58/79) and 62% (37/60), respectively. The difference in the concordance rate (73% versus 62%) was not statistically significant (P = .57). Among 21 patients in whom MR imaging was not concordant with PET, MR imaging correctly lateralized the lesion in six patients and PET in 12 patients (Fig 2). Among 23 patients in whom MR imaging was not concordant with ictal SPECT, MR imaging correctly lateralized the lesion in nine patients and ictal SPECT in 11 patients.
Correct lateralization rates in good outcome group (Engel's class I and II, n = 96)
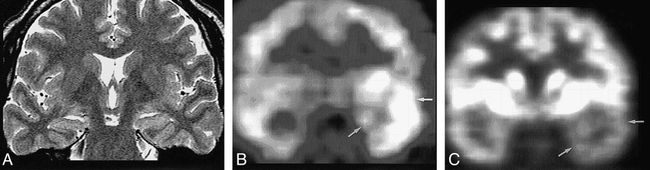
Nonconcordance among MR imaging, ictal SPECT, and PET in a 31-year-old woman with right temporal lobe epilepsy.
A, Oblique coronal turbo spin-echo T2-weighted MR image (5000/100/3) shows atrophy and slight hyperintensity of left hippocampus (arrow).
B, Ictal SPECT scan shows slight hyperperfusion in right temporal lobe (arrows). Radioactivity ratio between right temporal cortex/left temporal cortex was 1.2:1.
C, FDG-PET scan shows slight hypometabolism in right temporal lobe (arrows). Radioactivity ratio between right temporal cortex/left temporal cortex was 0.5:1.
Invasive EEG (subdural grids) study showed the epileptogenic focus in the right temporal lobe. After right anterior temporal lobectomy, pathologic diagnosis was hippocampal sclerosis combined with microscopic cortical dysplasia. Engel's outcome at 1-year follow up was class II.
When the preoperative MR imaging findings were compared with Engel's outcome class, 93% (53/57) of the patients with hippocampal sclerosis as seen on MR images, 74% (26/35) of the patients with a focal lesion other than hippocampal sclerosis (tumor, vascular malformation, focal cerebromalacia, etc), and 65% (17/26) of the patients with normal MR imaging findings were included in the good outcome group (Engel's class I or II, n = 96) (Table 5). In 17 patients who had both normal MR imaging findings and good outcome (class I or II), PET and ictal SPECT correctly lateralized the lesion in 85% (11/13) and 75% (9/12), respectively (Fig 3).
Preoperative MR imaging diagnosis versus good outcome rate (Engle's class I and II)
False-negative MR findings in a 30-year-old man with left complex partial seizures. Ictal activity was shown in left temporal area on video/EEG.
A, Oblique coronal fast spin-echo T2-weighted MR image (4000/120/4) shows no abnormalities.
B, Ictal SPECT scan shows hyperperfusion in left temporal lobe (arrows). Radioactivity ratio between right temporal cortex/left temporal cortex was 1:2.4.
C, FDG-PET scan shows hypometabolism in left temporal lobe (arrows). Radioactivity ratio between right temporal cortex/left temporal cortex was 1:0.7.
Invasive EEG showed ictal activity in left temporal lobe. After left anterior temporal lobectomy, pathologic diagnosis was hippocampal sclerosis of a mild degree associated with mild cortical dysplasia. Engel's outcome was class II.
Discussion
Although EEG remains the backbone for the evaluation of epilepsy, MR imaging currently is the method of choice for depicting gross structural lesions in the brain. PET and SPECT play a complementary role in the localization or lateralization of the epileptogenic focus and minimize the need for invasive EEG monitoring (7). In our study, the nonconcordance rate in lateralization between ictal video/EEG and MR imaging was approximately 30% to 40% of patients. This discrepancy necessitates the use of multiple imaging techniques for accurate presurgical evaluation.
The nonconcordance rate among the different imaging techniques is also high, by as much as 30% to 40%, as seen in our study. The neuroimaging techniques measure different aspects of the epileptic process; that is, structure (MR imaging), metabolism (PET), and perfusion (SPECT). MR imaging depicts only gross anatomic alterations associated with epilepsy. Anatomicopathologic definition of the organic lesion is the most obvious on MR images. PET has the unique ability to image cerebral metabolism but is virtually limited to the interictal state, because it takes approximately 1 hour for the radiotracer to enter the cells to be metabolized and to be distributed throughout the brain tissue. This time is too long to image the relatively short ictal state. If PET is performed immediately after cessation of seizure activity, with immediate injection of the radiotracer during the seizure, the PET image shows poor uptake of the radiotracer throughout the brain. A PET scan obtained 60 minutes after injection of the radiotracer during the seizure mostly reflects the postictal or interictal state. SPECT, on the other hand, is used to assess cerebral blood flow changes not only in the interictal state but also during the ictal period, as the radiotracer injected during the ictal state is distributed throughout the brain tissue within 1 minute and remains radioactive in the brain tissue after cessation of seizure activity without significant redistribution, thus enabling interictal scanning of the “ictal images.” An interictal SPECT scan may show decreased regional cerebral perfusion in patients with epilepsy, but its sensitivity is significantly lower than that of ictal SPECT and interictal PET in identifying a focal deficit in patients with partial seizures (5, 8, 9). In our experience, interictal SPECT had a very low diagnostic yield (sensitivity of less than 50%) in daily practice. This is why we did not include interictal SPECT in our study, although it was performed in 110 patients as a presurgical examination.
According to the literature review conducted by Spencer and colleagues (10, 11), the highest sensitivity was achieved by ictal SPECT (90% in temporal lobe epilepsy, 81% in extratemporal epilepsy). Among the interictal techniques, PET showed the highest sensitivity in patients with temporal lobe epilepsy (84% versus 66% for interictal SPECT, 55% for qualitative MR imaging), while interictal SPECT showed the highest sensitivity in patients with extratemporal epilepsy (60% versus 43% for MR imaging and 33% for PET). As in other studies, our investigation suggests that all imaging techniques had a lower sensitivity for extratemporal epilepsy than for temporal lobe epilepsy. Markand et al (12) reported that the sensitivity of ictal SPECT was almost equal to that of PET (86% versus 86%) in patients with complex partial seizures. In our study, PET showed the highest sensitivity for both temporal and extratemporal lesions. Unlike the results of the previous studies, PET was superior to ictal SPECT (85% versus 73%). In patients with normal MR imaging findings, PET was also superior to ictal SPECT in lateralizing the lesion (80% versus 55%). In ictal SPECT, prompt injection of the radiotracer appears to be a critical factor, since there is a rapid evolution of the regional cerebral blood flow from the ictal to the early and late postictal states, which may vary from patient to patient. If the radiotracer is injected late during the seizure or after the seizure is over, isoperfusion or even hypoperfusion may be seen in the responsible area (13). Because the spread of ictal discharges to other areas of the brain may occur within seconds of seizure onset, the SPECT scan, even with injection approximately 1 minute after the onset of a seizure, may not indicate the site of onset. In our study, the relatively lower sensitivity of ictal SPECT might have been due to either relatively late injection or individual variation in the evolution of the regional cerebral blood flow. Relatively higher resolution of PET, compared with SPECT, might be another factor that contributed to the higher sensitivity of PET. Ictal SPECT, however, showed sensitivity comparable to MR imaging in localization of epileptic foci and can be used as a reliable alternative, especially when PET is unavailable. The discrepancy in sensitivity of MR imaging between the previous studies and our study might reflect the fact that the previous studies were published during a time of technical maturation of MR imaging. If we had used the results of retrospective, blinded reinterpretations of the images instead of the original reports, the concordance rate and sensitivity would have been higher than the current results, as there would be an intraindividual learning curve that would make current interpretation more effective.
In our opinion, in patients in whom a definite unilateral lesion is found on MR images that is concordant with scalp ictal video/EEG, the functional imaging techniques (PET and ictal SPECT) do not appear to provide additional useful information. On the other hand, in patients who have inconclusive or normal MR imaging findings or in whom there is nonconcordance between ictal EEG and MR imaging, PET or ictal SPECT is valuable in weighing further diagnostic or therapeutic options. It is difficult to ascertain whether two functional imaging techniques (PET and ictal SPECT) are redundant or complementary to each other for localizing information. Our data suggest that these two techniques are complementary.
Good outcomes (Engel's class I [seizure-free] and II [rare seizures, meaning a few in a year]) after surgery for temporal lobe epilepsy have been reported in approximately 70% to 90% of cases (14, 15). Kilpatrick et al (7) reported a seizure-free rate (class I) of 78% in 50 patients who had undergone temporal lobe epilepsy surgery. Similar results were reported after extratemporal lesionectomies (16). The results of extratemporal, nonlesional surgery were clearly worse, with class I and II rates of only 30% to 40%. Outcomes in our study were similar to those reported in previous investigations (7, 16). The nature of the abnormality, as revealed by MR imaging, is an important factor in postoperative outcome. Garcia et al (17) reported a better outcome in patients with unilateral hippocampal atrophy (96% seizure-free) than in patients with normal MR imaging findings (50% seizure-free). In our study, 93% of the patients with MR findings of unilateral hippocampal sclerosis and 65% of the patients with normal MR findings were seizure-free (class I). Jack et al (18) also reported the best outcome in patients with hippocampal atrophy on MR imaging. Similar results have been reported by others (19–21).
Our study is limited by the relatively short follow-up period; hence, it is necessary to be cautious as to the long-term prognosis for these patients. Berkovic et al (13) found that the percentage of patients achieving a 2-year seizure-free period after temporal lobectomy changes with time, and that patients with hippocampal sclerosis are at particular risk for late recurrence of seizures. In a recent series of 135 patients followed up for 5 years after temporal lobectomy, 69% with foreign tissue lesions (tumors and vascular malformations), 50% with hippocampal sclerosis, and 21% with normal MR findings had no postoperative seizures (13).
Conclusion
The overall concordance rate among the various imaging methods, including ictal video/EEG, MR imaging, PET, and ictal SPECT, is approximately two thirds or less in lateralizing the epileptogenic focus. Each technique has its own advantages, and they play complementary roles. PET is the most sensitive in lateralization of the epileptogenic area, even though it provides an approximate location of the epileptogenic zone, which by itself is not adequate for epilepsy surgery. PET and/or ictal SPECT may well be complementary tools in patients with inconclusive lateralization on ictal video/EEG and MR imaging.
Footnotes
↵1 Supported by a grant from Medical Technology Development Research Fund, Ministry of Health and Welfare, Seoul, Korea (HMP-96-M-2-1039).
↵2 Address reprint requests to Kee-Hyun Chang, MD, Department of Diagnostic Radiology, Seoul National University Hospital, 28 Yeongon-dong, Chongno-gu, Seoul 110-744, Korea.
References
- Received August 11, 1998.
- Copyright © American Society of Neuroradiology