Abstract
BACKGROUND AND PURPOSE: The posterior cricoarytenoid (PCA) muscle is one of the intrinsic muscles of the larynx innervated by the recurrent laryngeal nerve. As such, recurrent laryngeal nerve palsy should not only result in paralysis of the true vocal cord or thyroarytenoid muscle but also in a similar change in the PCA muscle. The ability of CT and MR imaging to depict denervation atrophy in the PCA muscle in patients with recurrent laryngeal nerve palsy was evaluated.
METHODS: Two investigators reviewed the CT and/or MR studies of 20 patients with a clinical history of vocal cord paralysis. The appearance of the PCA muscle was given a rating of 0, 1, 2, 3, or 4, with 0 being definitely normal and 4 being definitely abnormal or atrophic. Each study was also reviewed for the presence or absence of other features of vocal cord paralysis: thyroarytenoid muscle atrophy, anteromedial deviation of the arytenoid cartilage, an enlarged piriform sinus and laryngeal ventricle, and a paramedian cord.
RESULTS: Atrophy of the PCA muscle was shown unequivocally in 65% of the cases and was most likely present in an additional 20%. The frequency with which other features of vocal cord paralysis were seen was as follows: thyroarytenoid atrophy, 95%; anteromedial deviation of the arytenoid cartilage, 70%; enlarged piriform sinus, 100%; enlarged laryngeal ventricle, 90%; and a paramedian cord, 100%.
CONCLUSION: Atrophy of the PCA muscle may be commonly documented on CT and MR studies in patients with recurrent laryngeal nerve palsy and vocal cord paralysis, and therefore should be part of the constellation of imaging features of vocal cord paralysis. This finding is particularly useful when other imaging findings of vocal cord paralysis are absent or equivocal.
The imaging features of vocal cord paralysis include atrophy of the thyroarytenoid muscle, anteromedial deviation of the arytenoid cartilage, enlarged laryngeal ventricle, enlarged piriform sinus, and a paramedian vocal cord (1–3). The posterior cricoarytenoid (PCA) muscle, an intrinsic muscle of the larynx, has sufficient bulk to be reliably identified on cross-sectional imaging studies. As such, atrophy of this muscle should also be detectable in a patient with vocal cord paralysis.
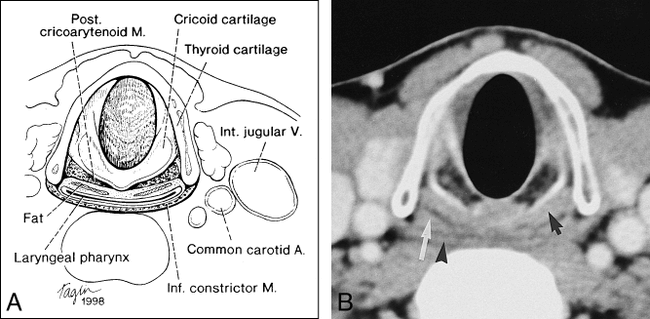
The PCA muscle, the only abductor of the vocal cords, is readily identifiable on cross-sectional CT and MR studies as a triangular muscle bundle along the posterior surface of the cricoid cartilage (Figs 1 and 2). This muscle arises along the posterior surface of the cricoid cartilage and extends superolaterally to insert on the muscular processes of the arytenoid cartilage (4, 5) (Fig 2). This muscle is innervated by the recurrent laryngeal nerve branch of the vagus nerve, the same nerve that innervates the thyroarytenoid muscle, which accounts for the bulk of the true vocal cord. The detection of atrophy of the PCA muscle on CT and MR examinations in a patient with a history of vocal cord paralysis may be helpful when other imaging features of vocal cord paralysis are absent or equivocal.
A, Axial depiction of the PCA muscle.
B, Equivalent axial CT scan (black arrow indicates PCA muscle; white arrow, collapsed laryngeal pharynx). Note thin black line of fat between muscle and pharynx (arrowhead indicates inferior constrictor muscle).
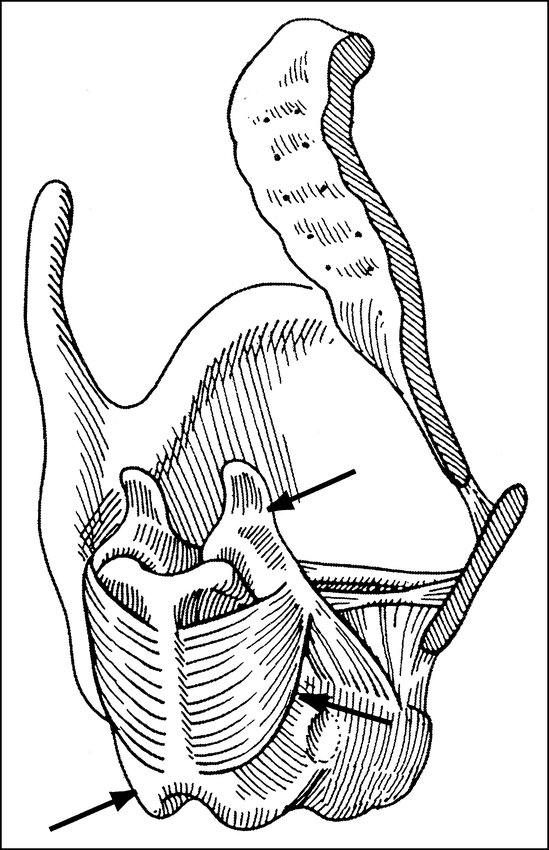
Diagram of the posterolateral view of the laryngeal skeleton (right thyroid lamina removed) shows the PCA muscle (middle arrow) arising from the cricoid lamina (lower arrow) posteriorly and inserting on the arytenoid cartilage (upper arrow). Reproduced from (5) with permission
In this study, we reviewed the CT and/or MR images of 20 patients with vocal cord paralysis, documenting the frequency of the various findings of vocal cord paralysis, with particular emphasis on our ability to detect atrophy of the PCA muscle.
Methods
Two radiologists independently reviewed the CT and/or MR studies of 20 patients with a clinical history of chronic (> 6 months) vocal cord paralysis. The patients ranged in age from 17 to 82 years; 12 were males and eight were females. The studies were performed between January 1995 and 1998. Fifteen patients had CT only, four patients had both CT and MR imaging, and one patient had MR imaging only. Of the 15 patients who had CT only, vocal cord paralysis was idiopathic in 11; in the remaining four, it was associated with thyroid malignancy, Pancoast tumor, metastatic cervical lymph nodes from adenocarcinoma of the breast, and aneurysm of the aortic arch, respectively. Of the four patients who had both CT and MR imaging, vocal cord paralysis resulted from glomus vagale tumors in two and from thyroid malignancies in two. Finally, in the one patient who had MR imaging only, vocal cord paralysis was associated with a vagal schwannoma.
All CT studies were performed with 100 mL of intravenous contrast material injected at a rate of 1.2 to 1.4 mL/s with a 40- to 50-second delay. Scans were acquired during quiet respiration, parallel to the laryngeal ventricle, from the skull base to the aortic arch using various spiral techniques: the section thickness or collimation varied from 2 to 5 mm, with a table speed of 3 to 5 mm/s; the reconstruction interval varied from 2 to 5 mm. The variation in technique was random. MR imaging was performed on a 1.5-T magnet. Axial T1-weighted (800/12 [TR/TE) and fat-saturated T2-weighted (4000/99) images from the skull base to the thoracic inlet were obtained at 5-mm intervals with a 30% to 35% skip between sections.
The position of the vocal cord on the side of the paralysis was documented in every study. As well, each study was evaluated for the presence or absence of atrophy of the thyroarytenoid and PCA muscles on the side of the paralysis relative to the normal opposite side. Atrophy of the thyroarytenoid muscle was defined as decreased muscle bulk with or without fatty change relative to the normal side and with or without distension of the laryngeal ventricle into the space otherwise occupied by normal muscle. Atrophy of the PCA muscle was defined as decreased bulk in the axial plane with or without fatty change relative to the normal side. A decrease in muscle bulk could be seen as shortening of the anterior to posterior dimension as well as an apparent increase in the amount of fat immediately posterior to the cricoid cartilage or anterior to the muscle. Atrophy of the PCA was given a rating of 0, 1, 2, 3, or 4, where 0 = definitely normal, 1 = probably normal, 2 = indeterminate, 3 = probably abnormal, and 4 = definitely abnormal. In addition, each study was evaluated for the presence or absence of anteromedial deviation of the arytenoid and an enlarged ventricle and piriform sinus on the side of the paralysis (Figs 3–6⇓⇓⇓).
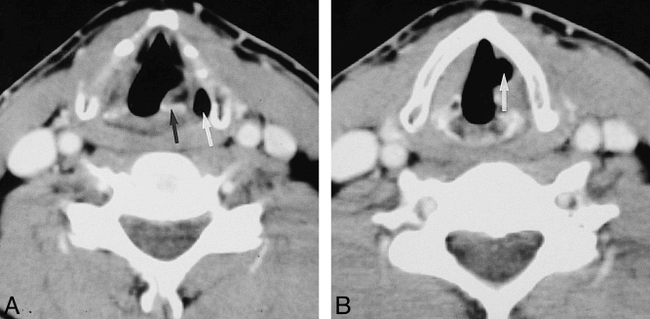
A, Axial CT scan in a patient with left vocal cord paralysis shows enlargement of the left piriform sinus (white arrow) and anteromedial deviation of the arytenoid cartilage (black arrow).
B, Axial CT scan in the same patient shows atrophy of the left thyroarytenoid muscle as evidenced by enlargement of the left laryngeal ventricle (white arrow) relative to the right.
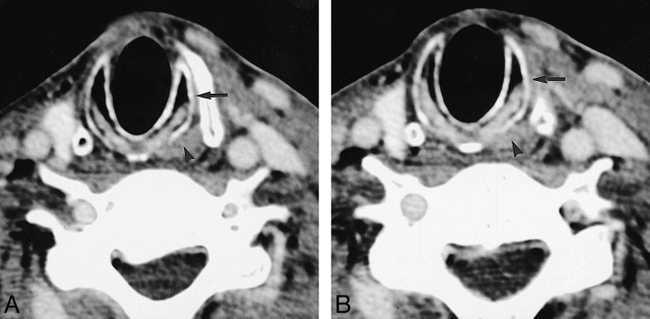
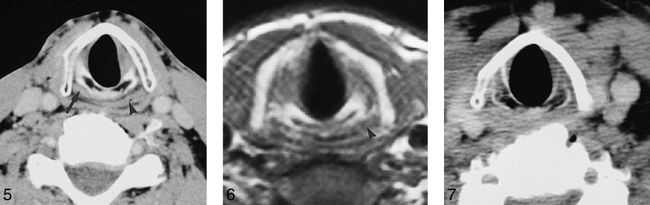
A and B, Two consecutive axial CT scans in a patient with a right-sided glomus vagale show atrophy of the right PCA muscle and right cricothyroid muscle relative to the normal left side (arrowhead indicates normal left PCA muscle; arrow, normal left cricothyroid muscle)
Axial CT scan in a patient with left vocal cord paralysis shows atrophy of the left PCA muscle (arrow indicates normal right PCA muscle; arrowhead, laryngeal pharynx).
fig 6. T1-weighted axial MR image (800/12) in a patient with a right-sided vagal schwannoma shows atrophy of the right PCA muscle relative to the left (arrowhead indicates normal left PCA muscle).
fig 7. Axial CT scan through the level of the PCA muscles in a patient with a left-sided vocal cord paralysis. Atrophy of the left PCA muscle is probably present (a rating of 3) although poorly seen, given the lack of fat posterior to it.
Finally, each study was assessed for atrophy of the cricothyroid muscle (Fig 4A and B). This muscle, like the thyroarytenoid muscle, is an intrinsic muscle of the larynx; however, it is not innervated by the recurrent laryngeal nerve but rather by the external branch of the superior laryngeal nerve. This nerve arises from the vagus at the level of the inferior or nodose ganglion close to the skull base (4).
Any imaging study for which there was a disagreement between the two readers was reviewed by the two together and a consensus was reached. The presence or absence of an individual finding was tallied by patient and not by imaging technique (eg, CT vs MR).
Results
The position of the paralyzed vocal cord in all 20 cases (100%) was found to be paramedian or intermediate between full abduction and adduction. Atrophy of the thyroarytenoid muscle was appreciated in 19 (95%) of 20 patients. A vocal cord injection precluded evaluation of thyroarytenoid atrophy in one patient.
Atrophy of the PCA muscle was seen as “definitely abnormal” (a rating of 4) in 13 (65%) of the 20 patients. Among these, the section collimation on CT was 2 or 3 mm in nine patients and 5 mm in four patients. In the remaining seven patients, the appearance of atrophy was rated 1 (n = 1), 2 (n = 2), or 3 (n = 4). In the six patients in whom the rating for PCA atrophy was 2 or 3, there was poor distinction between the PCA muscle and the laryngeal pharynx, with the apparent limiting factor being either a paucity of or a poor demonstration of the fat between the muscle and the laryngeal pharynx (Fig 7). The lesser ratings of 2 or 3 did not appear to be related to the variation in spiral imaging technique. In the four patients whose studies had a rating of 3, section collimation was 5 mm in two and 3 mm in the other two. Similarly, in the two patients whose studies had a rating of 2, one CT examination was performed with a section collimation of 3 mm and the other with a 5-mm collimation. In the one patient whose studies had a rating of 1, section collimation was 3 mm. Atrophy of the PCA muscle and an enlarged piriform sinus were the only findings of vocal cord paralysis in one patient.
Anteromedial deviation of the arytenoid cartilage was appreciated in 14 (70%) of the 20 patients. In the six patients in whom it was not appreciated, two had vocal cord implants, one had a vocal cord injection, and three had a paucity of ossification of the arytenoid cartilage itself.
In all 20 patients (100%), an enlarged piriform sinus was identified on the side of the vocal cord paralysis. An enlarged laryngeal ventricle was identified in 18 (90%) of the 20 patients. This finding was not apparent in one patient with a vocal cord injection and in another patient with a vocal cord implant.
Atrophy of the cricothyroid muscle was seen in one patient with a history of a glomus vagale. In the other two patients with vagal nerve lesions (schwannoma and paraganglioma), atrophy of the cricothyroid muscle was not seen.
In the four patients who had both CT and MR examinations, no discrepancy was noted in the findings between the two techniques.
Discussion
Many of the signs of vocal cord paralysis we appreciate today on CT and MR studies were first described with laryngography (1–3). These include atrophy of the thyroarytenoid muscle, deviation of the arytenoid muscle, enlargement of the ventricle and piriform sinus on the side of the paralysis, and a paramedian position of the involved vocal cord. As we determined, an additional CT and MR feature of vocal cord paralysis is atrophy of the PCA muscle.
Atrophy of the PCA and thyroarytenoid muscles usually occurs as a result of recurrent laryngeal or vagal nerve palsy. Muscular atrophy consequent to a nerve palsy is referred to as denervation atrophy. Denervation atrophy has been documented on CT and MR studies in skeletal muscles as well as in muscles of the head and neck innervated by various cranial nerves, including the trigeminal (V), facial (VII), vagus (X), spinal accessory (XI), and hypoglossal (XII) nerves (6–8). Imaging criteria for the diagnosis of denervation atrophy include asymmetric decrease in the affected muscle volume, fatty infiltration of the involved muscle group, and involvement of multiple commonly innervated muscles. The development of denervation atrophy is usually witnessed on imaging studies within a few weeks of injury to the nerve (6).
The detection of PCA atrophy was variable in our study. All our patients had chronic vocal cord paralysis of 6 months' duration or more and should, therefore, have developed PCA atrophy. We were able to unequivocally detect atrophy of this muscle in 65% of the patients. In an additional 20%, we were able to conclude with a high degree of probability that atrophy was present. The factor that seemed to limit our appreciation of PCA atrophy was fat. In the patients in whom atrophy was detected unequivocally, a clear curvilinear margin of fat was appreciated between the posterior surface of the PCA muscle and the anterior surface of the laryngeal pharynx. As such, the bulk of the muscle could easily be compared with that on the normal side. In patients in whom there was a paucity or absence of this fat, the posterior surface of the PCA muscle could not clearly be separated from the anterior surface of the pharynx. With the two being of similar density/intensity, PCA atrophy could not be detected with complete certainty. Other factors that could potentially influence the detection of PCA atrophy include section collimation, the size of the patient, and the presence of degenerative cervical spine disease. A statistical analysis of the relationship between section thickness and PCA atrophy was not performed, although, in our study, there seemed to be as much variability in technique in patients in whom atrophy was appreciated as in those in whom it was not. In large patients, streak artifacts from the shoulders could make it difficult to distinguish soft tissues posterior to the cricoid cartilage. Cervical spine osteophytes could produce beam-hardening artifacts, which also would result in suboptimal visualization of the PCA muscle.
The demonstration of PCA atrophy is clinically useful. First, the clinician and/or radiologist may not know or suspect that there is vocal cord paralysis, and the other imaging features of vocal cord paralysis may be equivocal. For example, the laryngeal ventricle may appear enlarged without paralysis, and the thyroarytenoid muscle may not be seen in its entirety, making the detection of atrophy difficult. Under these circumstances, detection of atrophy of the PCA muscle would imply that there is vagal or recurrent laryngeal nerve compromise and, most likely, vocal cord paralysis. Second, the clinical distinction between vocal cord fixation and paralysis may not be obvious. In a patient who has hoarseness, the vocal cord may be fixed and immobile or, alternatively, paralyzed. Fixation is caused by mechanical interference, and paralysis is caused by a neurologic compromise. Fixation may be due to tumor infiltration of the thyroarytenoid muscle or to a large tumor that interferes with cord movement. Similarly, arthritis of the cricoarytenoid joint related to prior traumatic intubation or to connective tissue disease may render the cord immobile. Paralysis may be due to intrinsic lesions of the recurrent laryngeal nerve or vagus nerve, extrinsic lesions that compress and compromise the function of these nerves, or surgical ligation of either nerve. As such, a fixed cord should not be accompanied by atrophy of the PCA muscle, and a paralyzed cord should be accompanied by atrophy.
Other previously described imaging features of vocal cord paralysis were observed with variable frequency in our series. The paramedian, intermediate, or cadaveric position of the paralyzed vocal cord, a position that is slightly lateral to midline, was evident in all our patients and has an anatomic basis. The recurrent laryngeal nerve supplies both the abductors and adductors of the cord. Thus, when compromised, the cord most commonly lies between abduction and adduction (9). Thyroarytenoid atrophy was also commonly observed in our series and was documented by a decrease in muscle bulk or fatty infiltration (1). Although commonly observed, it is worth remembering that atrophy of the thyroarytenoid muscle is frequently a subtle finding. The degree of atrophy is often slight and difficult to appreciate in the axial plane, because, frequently, an image through the center of the cord may not be obtained. An enlarged laryngeal ventricle was also an indication of cord atrophy, as it distended into the space created by decreased muscle bulk of the true cord. The presence of one or more of these findings is likely to depend on the duration and degree of recurrent laryngeal nerve paralysis.
In contrast, anteromedial deviation of the arytenoid cartilage was not as constant a finding. This position is caused by anteromedial rotation of the arytenoid muscle in vocal cord paralysis (1). In our series, this finding was not evident in six patients, three with vocal cord implants and injections and three in whom the arytenoids were poorly visualized, most likely because of a paucity of ossification. However, piriform sinus dilatation is a direct result of anteromedial deviation of the arytenoid, as the arytenoid cartilage and aryepiglottic fold form the medial border of the piriform sinus. Therefore, since piriform sinus dilatation was observed in all the patients, some degree of arytenoid deviation was most likely present, albeit not detectable by imaging.
Our experience with the efficacy of CT and MR imaging in revealing atrophy of the cricothyroid muscle in patients with vocal cord paralysis was limited. Since this muscle is innervated by the superior laryngeal nerve, atrophy of this muscle would only be anticipated with proximal vagal nerve lesions. In our series, only three patients had such lesions, and in only one was atrophy of this muscle identified. Identifying atrophy of the cricothyroid muscle may be clinically useful. In some patients with hoarseness and presumed vocal cord paralysis, the clinician may be unable to ascertain whether the pathologic condition is proximal (vagal) or distal (recurrent laryngeal nerve). Theoretically, patients with proximal vagal nerve disease should not only have hoarseness or vocal cord paralysis but also oropharyngeal signs and symptoms. These include uvular deviation, loss of gag reflex, and atrophy of the soft palate and superior constrictor muscle. These patients might also have other cranial nerve neuropathies, namely, glossopharyngeal (IX), spinal accessory (XI), and hypoglossal (XII) disturbances, because of their common proximal course with the vagus nerve (10). However, oropharyngeal signs and symptoms and other cranial neuropathies may be absent or equivocal. In this situation, the identification of cricothyroid atrophy would indicate proximal disease, and its absence, distal disease. However, the ability of imaging to depict atrophy of this muscle remains to be determined.
Conclusion
Atrophy of the PCA muscle may commonly be appreciated on CT and MR studies of patients with vocal cord paralysis. Demonstration of atrophy of the PCA muscle is a useful confirmatory imaging feature of vocal cord paralysis, especially when other features are absent or equivocal. Such features include thyroarytenoid atrophy, anteromedial deviation of the arytenoid cartilage, an enlarged piriform sinus and laryngeal ventricle, and a paramedian cord.
Footnotes
↵1 Address reprint requests to Laura Vitale Romo, MD, Department of Radiology, Massachusetts Eye and Ear Infirmary, 243 Charles St, Boston, MA 02114.
- Received August 24, 1998.
- Copyright © American Society of Neuroradiology