Abstract
BACKGROUND AND PURPOSE: A number of anatomicoclinical syndromes have been described in which bilateral symmetrical polymicrogyria is the underlying morphologic abnormality. We retrospectively reviewed the clinical, epileptic, and morphologic manifestations of bilateral symmetrical polymicrogyria in 21 patients to determine whether certain areas are at particular risk for these syndromes.
METHODS: Clinical records and brain MR studies of 21 patients with bilateral symmetrical polymicrogyria were reviewed to confirm the presence and determine the location of polymicrogyria and to qualitatively correlate location with developmental, neurologic, and epileptic histories. The locations we found were compared with published reports of bilateral symmetrical polymicrogyria to determine whether these locations were random or whether predilections exist for certain areas.
RESULTS: Analysis revealed six patients with bilateral frontal polymicrogyria, nine with bilateral perisylvian polymicrogyria, one with bilateral parietal polymicrogyria, one with bilateral parasagittal parieto-occipital polymicrogyria, two with bilateral frontal polymicrogyria and bilateral perisylvian polymicrogyria, one with bilateral perisylvian and bilateral parasagittal parieto-occipital polymicrogyria, and one with bilateral perisylvian, bilateral parieto-occipital, and bilateral parasagittal parieto-occipital polymicrogyria. Symptom complexes were nonspecific, but seemed additive according to the regions of brain involved.
CONCLUSION: Bilateral symmetrical polymicrogyria has a propensity to develop in specific regions of the cerebral cortex. When the regions are extensive, the areas involved often appear to be simple topological additions of those regions. These locations and the identification of several familial cases raise the possibility that genetic mechanisms influence the development of these malformations in some patients.
In the past 12 years, our knowledge about malformations of cortical development has grown tremendously. Major contributions to this growth have come from the advent of modern neuroimaging (1–11) and modern molecular genetics (12–15). Recently, several syndromes have been described in which patients have rather specific clinical manifestations associated with imaging findings of bilateral symmetrical polymicrogyria. These include bilateral perisylvian polymicrogyria (4, 16–18), bilateral parasagittal parieto-occipital polymicrogyria (19, 20), and bilateral frontal polymicrogyria (R.G., unpublished observations). Instances of bilateral polymicrogyria have generally been considered sporadic, although some familial cases have been reported (4, 16). The increasing number of reported locations of bilateral symmetrical polymicrogyria prompted a review of the imaging studies of all patients seen at our institution to confirm the presence and determine the location of the polymicrogyria and to correlate qualitatively the location with developmental, neurologic, and epileptic histories. It was hoped that a better understanding of the locations involved might aid in determining whether these malformations are the result of genetic influences or of intrauterine insult.
Methods
A review of the teaching files and the radiologic information system at our institution yielded 21 patients with MR imaging findings of bilateral symmetrical polymicrogyria. Twelve of these cases have previously been reported in articles describing specific bilateral polymicrogyria syndromes (16, 19). Eleven patients were female, and 10 were male (see Table 1). Their ages ranged from 6 months to 32 years at the time of their most recent clinical examination, with a mean age of 6 years and a median age of 2½ years. Their MR studies were performed within a few days to 2 years from the time of their most recent clinical examination.
Findings in 21 patients with polymicrogyria
The MR studies and clinical records of these patients were examined retrospectively to confirm the presence and determine the location of the polymicrogyria. The images were initially evaluated, and the diagnosis made, by a number of different neuroradiologists. All were reviewed again by one of the authors to ensure that the diagnosis was correct. The diagnosis of bilateral symmetrical polymicrogyria was confirmed if regions of the cortex in the same area of both hemispheres were subjectively judged as showing an abnormal gyral pattern, increased cortical thickness, and irregularity of the cortical–white matter junction. These criteria were met in all 21 patients. The locations of polymicrogyria were then qualitatively correlated with developmental, neurologic, and epileptic histories, which were obtained from the clinical records. Furthermore, the locations of polymicrogyria were studied and correlated with previously published reports of bilateral symmetrical polymicrogyria in an attempt to determine whether these locations were random or whether predilections exist for certain areas.
Because the MR studies were performed over a 13-year period and at several institutions, the imaging sequences varied considerably. All patients had sagittal T1-weighted and axial T2-weighted studies. Fifteen patients had axial T1-weighted studies. Seven patients had coronal T1-weighted studies, and four had coronal T2-weighted studies.
The medical records were scrutinized to assess motor and cognitive development, neurologic status, and the presence of epilepsy (and, if present, the age of onset, seizure semiology, and underlying EEG abnormality, if any). EEG recordings were performed using the 10–20 International Electrode Placement System. Epileptic seizures were classified according to the recommendations of the International League Against Epilepsy (21).
Results
Morphology
The results are summarized in the Table. Six patients had polymicrogyria extending from the frontal poles anteriorly to the precentral gyrus posteriorly (Fig 1); the frontal operculum was involved and marked the inferior extent in almost all cases. These patients were classified as having bifrontal polymicrogyria. Nine patients had polymicrogyria involving the frontal, parietal, or temporal opercula; these patients were classified as having bilateral perisylvian polymicrogyria, and were further classified into those in whom nearly the entire perisylvian cortex was affected (termed holosylvian polymicrogyria (n = 3; Fig 2) and those in whom only the posterior perisylvian cortex was involved (termed posterior perisylvian polymicrogyria (n = 6; Fig 3). Two patients had polymicrogyria involving most of both frontal lobes and the entire perisylvian cortex; these patients were classified as having bilateral frontal and sylvian polymicrogyria (Fig 4). One patient had polymicrogyria limited to the parietal cortex, sparing the perisylvian region (Fig 5), and was classified as having bilateral parietal polymicrogyria. Another had polymicrogyria in the parasagittal plane, involving the parietal and occipital lobes (Fig 6); this pattern has been termed bilateral parasagittal parieto-occipital polymicrogyria (19). One patient had polymicrogyria involving the posterior perisylvian cortex and extending posteriorly and medially into the parasagittal parieto-occipital region (Fig 7). The final patient had polymicrogyria involving the entire perisylvian cortex, extending posteriorly into the lateral parietal lobes and posteromedially into the parasagittal parietal and occipital lobes (Fig 8); this pattern was termed bilateral holosylvian, lateral parietal, and parieto-occipital polymicrogyria.
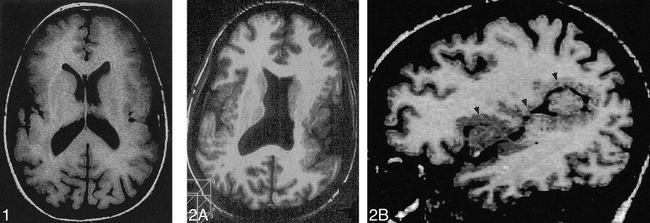
Case 1: 3-year-old girl with bifrontal polymicrogyria. Axial spin-echo (SE) (600/20) MR image shows shallow sulci with irregularity or the cortical–white matter junction, consistent with polymicrogyria, involving the entire frontal cortex posteriorly to the central sulcus.
fig 2. Case 15: 10-month-old girl with bilateral holosylvian polymicrogyria.
A, Axial reformation from 3D Fourier transformation (3DFT) gradient-echo (GRE) (35/7) image shows thickened, irregular cortex involving the entirety of the cortex surrounding the sylvian fissures and widening of the fissures.
B, Sagittal reformation from 3DFT GRE (35/7) image shows that the entire perisylvian cortex (arrows) is abnormal.
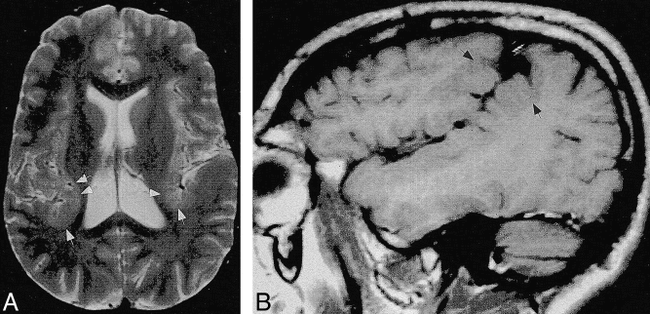
Case 12: 31-year-old man with bilateral posterior sylvian polymicrogyria.
A, Axial SE (2500/80) image shows normal anterior perisylvian cortex, with thickening of the cortex (arrows) posteriorly.
B, Sagittal SE (600/20) image shows that the abnormal posterior perisylvian cortex extends superiorly (arrows) to the parietal convexity.
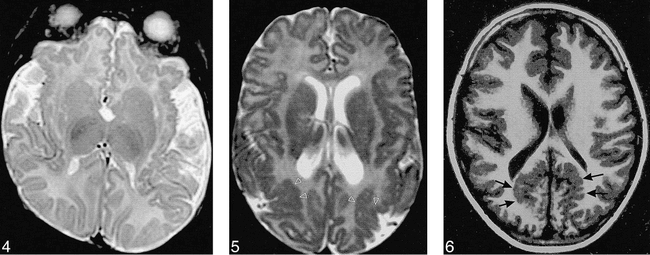
Case 19: 14-month-old boy with bilateral frontal and sylvian polymicrogyria. Axial SE (3000/120) image, obtained at age 3 months, shows polymicrogyria involving the orbital and medial surfaces of the frontal lobes and along the insular cortex. The opercula are too wide.
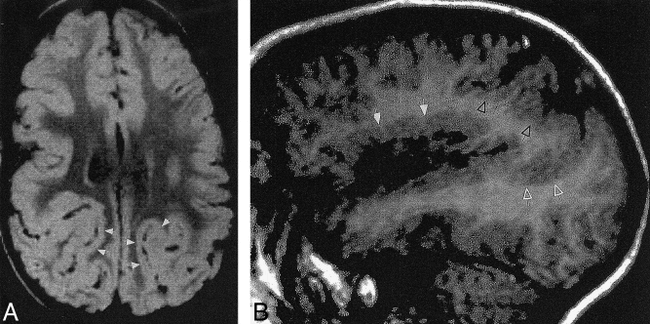
fig 5. Case 16: 10-month-old girl with bilateral lateral parietal polymicrogyria. Axial SE (3000/120) image shows polymicrogyria over the parietal convexities (arrows) bilaterally.
fig 6. Case 17: 6-year-old boy with bilateral parasagittal parieto-occipital polymicrogyria. Axial inversion-recovery (1600/16, IR = 400) image shows the irregular cortex (arrows) in a parasagittal location involving the parietal and occipital lobes.
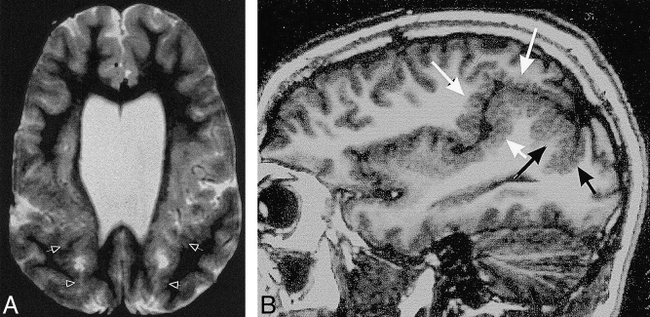
Case 20: 6-year-old boy with bilateral perisylvian and parasagittal parieto-occipital polymicrogyria.
A, Axial SE (2800/80) image shows polymicrogyria (arrows) involving the posterior perisylvian cortex and extending posteriorly and medially into the parietal parasagittal region.
B, Sagittal SE (550/11) image shows polymicrogyria continuing from the posterior sylvian area (white arrows) into the parieto-occipital area (black arrows).
Case 21: 7-year-old girl with bilateral perisylvian, lateral parietal, and parieto-occipital polymicrogyria.
A, Axial SE (2000/40) image shows polymicrogyria (arrows) involving the parasagittal parieto-occipital cortex and the lateral parietal cortex.
B, Sagittal SE (600/20) image shows the polymicrogyria involving the entire perisylvian cortex (solid white arrows) and extending posteriorly into the parietal (open black arrows) and occipital (open white arrows) lobes.
Developmental and Neurologic Function
The developmental patterns and neurologic function of the patients were as expected for the location and extent of the polymicrogyria (4, 18, 19, 22). Almost all patients in this series were delayed in achieving milestones, both motor and verbal. The patients with the relatively small regions of polymicrogyria had comparatively mild deficits while those with larger areas of polymicrogyria had more severe neurologic and developmental deficiencies.
As has been reported previously (4, 16, 18), patients with involvement of the perisylvian cortex had problems with phonation and delayed speech; most of the patients also had motor delays in infancy, often progressing to frank paraparesis or quadriparesis in the older patients. Those with bilateral frontal polymicrogyria showed nearly uniform quadriparesis with mild mental retardation; motor delay was more severe than speech delay. The two children with relatively limited parietal or parieto-occipital polymicrogyria had relatively minor motor dysfunction. Of these two patients, the 6-year-old with parieto-occipital polymicrogyria showed significant cognitive delay, whereas the 10-month-old with lateral parietal polymicrogyria was too young for cognition to be adequately assessed. The patients with extensive regions of polymicrogyria in each hemisphere all had significant neurologic and developmental problems, including hypotonia, hyperreflexia, contractures, and mental retardation. None of the oldest three patients (cases 18, 20, and 21) ever spoke more than four words.
Epilepsy
Seizures were reported in 10 of the 21 patients; nine of these had epilepsy, whereas one had febrile seizures and evidence of electrical dysfunction in the cerebral cortex (focus of abnormal slow waves). When seizure frequency was analyzed according to location of polymicrogyria, it was found that seizures were less common in the patients with bifrontal and bisylvian polymicrogyria than in those with parietal or more extensive malformations. None of the six patients with bifrontal polymicrogyria had seizures, whereas four of the nine patients with bilateral perisylvian polymicrogyria (two of six with posterior sylvian polymicrogyria and two of three with holosylvian polymicrogyria) had seizures. Seizures were identified in all patients with polymicrogyria involving the parietal lobes and in all patients with polymicrogyria involving more than one region.
Of the 10 patients with seizures, eight had partial seizures: seven with partial complex seizures and one with simple partial seizures.
The presence or absence of seizures seemed to be more closely related to the location of the polymicrogyria than to the age of the patient, although this finding may be related to the fact that many of the patients in our series were still very young at the time of their most recent examination (see Table).
Discussion
Polymicrogyria is a malformation of cortical development that is characterized by abnormal arrangement and excessive folding of cerebral cortical cell layers, often with fusion of the gyral surfaces (23). It is thought to result from abnormal organization of neurons within cortical lamina after completion of neuroblast migration from the germinal zone and through the intermediate zone of the developing brain (1, 24). Although at one time there was concern that polymicrogyria could not be accurately diagnosed by imaging (22), more recent work has shown that the diagnosis can be made confidently if irregularity of the cortical–white matter junction is detected by thin-section MR imaging (25). We used the combination of three characteristics to identify polymicrogyria: abnormal gyral pattern, increased cortical thickness, and irregularity of the cortical–white matter junction.
In this study, we reviewed the clinical features and topology of polymicrogyria in 21 patients with bilateral, symmetrical involvement of the cerebral hemispheres. Our patients had topological distribution of polymicrogyria in several discrete regions, some previously described: the perisylvian region (4, 18), the parasagittal parieto-occipital region (19), the frontal lobes, and the lateral parietal lobes. Several patients had polymicrogyria in areas that seemed to be combinations of the above-mentioned discrete regions. For example, patients 18 and 19 had involvement of both bilateral frontal and bilateral sylvian regions (Fig 4), whereas patient 20 had polymicrogyria of the posterior perisylvian and parasagittal parieto-occipital cortex (Fig 7), and patient 21 had involvement of the bilateral sylvian regions, the bilateral lateral parietal lobes, and the bilateral parasagittal parieto-occipital regions (Fig 8).
The location and topological extent of the polymicrogyria were often better determined from the sagittal images than from the axial or coronal images. The different angulations of the axial images sometimes gave misleading impressions as to the actual site (parietal versus occipital, middle sylvian versus posterior sylvian) of the polymicrogyria. We strongly recommend acquisition of thin-section (3- or 4-mm) sagittal images through the entire brain in patients with suspected polymicrogyria.
Symptom complexes were similar among patients with comparable morphology and seemed to overlap when the topological cortical involvement overlapped. The patients with more limited cortical involvement tended to have less severe clinical courses. The patients with bilateral perisylvian polymicrogyria all had speech delay or dysfunction, although this varied from mild to severe. More severe clinical manifestations, consisting of either profound developmental delay or difficulties with palatal and tongue movements, were seen in some. These abnormalities have been described previously in the context of bilateral perisylvian polymicrogyria (4, 18). Speech and motor delay were present in patients with more extensive (holosylvian) polymicrogyria and in those with more limited posterior perisylvian polymicrogyria, but those with holosylvian involvement had more severe disabilities than those with more focal involvement. The patients with bifrontal polymicrogyria who were adequately assessed all had spastic quadriparesis, delayed motor and language milestones, and mild mental retardation. None had the oromotor involvement seen in the children with bilateral perisylvian involvement. No patient with bifrontal polymicrogyria had any history of seizures. Both the patients with parietal polymicrogyria (one with lateral parietal and one with parasagittal involvement) had medically refractory epilepsy, but motor signs and symptoms were minimal or absent. The younger patient (with lateral parietal involvement) had significant developmental delay, and the older one (with medial parasagittal involvement) had moderate mental retardation.
Not surprisingly, patients with more extensive cortical involvement seemed to have the most severe clinical course. Those with both frontal and perisylvian involvement were severely globally delayed and hypotonic, had severe appendicular motor dysfunction, and had the oromotor dysfunction characteristic of the patients with perisylvian involvement. Patients 20 and 21, with perisylvian, parietal, and occipital involvement, had oromotor dysfunction, epilepsy, and moderate mental retardation, but only mild appendicular motor dysfunction. Thus, in this small series, it seemed that the motor and cognitive deficits were additive in accordance with the topological additions.
An interesting feature of this series and other published series of bilateral symmetrical polymicrogyria is the observation that the polymicrogyria in these patients seems to have consistent topological boundaries in the brain. The bifrontal polymicrogyrias consistently ended at the central sulcus posteriorly and at the sylvian fissure inferiorly. In parasagittal parieto-occipital polymicrogyria, the abnormal cortex extends from the occipital cortex just below the parieto-occipital sulcus (upper margin of the cuneus) to immediately behind the precuneus and superior parietal lobule (19). In bilateral perisylvian polymicrogyria, the abnormality consistently involves the frontal, temporal, or parietal operculum and adjacent parietal cortex (18, 26). In our series, one patient (case 16) had involvement of the parietal cortex spanning the region between the posterior aspect of the bilateral perisylvian polymicrogyria and the anterior aspect of the parasagittal parieto-occipital polymicrogyria. Patients 18 and 19 had polymicrogyria in regions that seemed to be simple additions of bifrontal and bilateral perisylvian involvement. The topology of involvement in patient 20 was like a summation of perisylvian and parasagittal parieto-occipital involvement, while that of patient 21 was equal to a summation of bilateral perisylvian, bilateral parietal, and bilateral parasagittal parieto-occipital polymicrogyria.
This series further suggests a striking predilection for involvement of perisylvian and/or frontal regions in persons with bilateral symmetrical polymicrogyria. Nineteen of the 21 cases in our series involved the perisylvian and/or frontal regions, and only four involved the parietal or occipital cortex (two patients had involvement of both areas). Reports of bilateral polymicrogyria in the literature likewise reflect a preponderance of cases involving the perisylvian or frontal cortex.
A number of different explanations could account for the patterns of polymicrogyria seen in our patients. Classically, polymicrogyria has been thought to result from ischemic injury (19, 23, 27). Moreover, several cases of bilateral perisylvian polymicrogyria are reported in patients who suffered presumed ischemic events in utero (28–30), and neuropathologic evidence of hypoxic-ischemic injury has been observed in association with bilateral perisylvian polymicrogyria (31, 32). Therefore, it is tempting to attribute all of the bilateral polymicrogyrias to intrauterine hypotension or vascular occlusions (arteries or veins). Although the perisylvian cortex is in the distribution of the middle cerebral artery, the patterns observed in our patients do not closely resemble those of classic vascular distributions. One possible explanation for the observed patterns of injury would be differential vulnerability of certain regions of developing cortex at different stages of development. The entire cerebral cortex does not develop simultaneously. The ventrolateral areas of the cortex develop early and the dorsomedial regions develop late (33). Indeed, studies in rhesus monkeys have shown that the neurons destined for the anterior cingulate cortex are generated 30 days earlier than the neurons for the visual cortex (34). It is also known that cell migration is dependent on the normally functioning ion channels and the N-methyl D-aspartate receptor mechanisms in the migrating cells (35). Thus, ischemia may affect actively migrating cells more than cells that are either preparing to migrate or those that have already reached the cortex. Another potentially vulnerable time may be the period of cellular proliferation, prior to migration. Algan and Rakic (36) were able to specifically injure dividing cells in the germinal zone by X-irradiation during the time of cell division. The result was a paucity of cells in the layer of cortex for which the dividing cells were predestined, a condition mimicking four-layered polymicrogyria (36).
The fact that three of our patients with perisylvian polymicrogyria are siblings strongly suggests a genetic contribution in at least some patients. Indeed, reports of familial cases (4, 16, 28, 37) are as common as those of prenatal ischemia (28–30). The candidate genes would most likely be those expressed in the developing cortex near the end of the period of neuronal migration, or, perhaps, in the germinal zone during periods of neuronogenesis. In the mouse, Emx1 is a gene expressed in virtually all layers of the developing cortex, with activity much greater in the posterior half of the cortex than in the anterior half (38); thus, mutation of Emx1 seems a potential candidate for a polymicrogyria gene. Tbr1 is another gene that is expressed in the developing cerebral cortex (39); mutation of Tbr1 in the mouse causes region-specific disorganization of cortical layers (R.H., unpublished results). It is likely that other genes that are expressed at critical periods of cerebral cortical development will be discovered and that these will be candidates for polymicrogyria genes as well. The development of “knockout” models, in which these genes are excised, may provide models to analyze further the mechanisms by which genetic polymicrogyria is formed. Studies of knockout rodent models, however, are limited in that malformations of the lissencephalic rodent cerebral cortex may be difficult to relate to malformations of the gyrencephalic human brain.
One final observation concerns the fact that the patients all tended to manifest signs and symptoms referable to the location of the polymicrogyria in their brains. The presence of neurologic deficits referable to these areas is a little surprising because it is generally accepted that the immature brain is not yet hardwired. Neonates who suffer substantial cortical infarctions often have minimal neurologic deficits; indeed, as many as 28% of neonates who suffer cerebral infarctions have no neurologic deficits whatsoever at age 5 years (40). This discordance between prenatal and postnatal lesions was also observed by Duchowny et al (41), who observed contralateral relocation of cortical function in children with early postnatal cerebral cortical injuries but no such relocation in children with developmental cortical lesions. Why, then, do the deficits persist in patients with presumed prenatal injuries? The answer is clearly beyond the scope of this article. One possibility, however, would be that the cortical layers that are malformed or damaged in polymicrogyria either spare the subplate or occur after its formation. The subplate is a transient cortical layer (sometimes called cortical layer 7 [33]) that exists during the period of cortical development (42). It has been shown that the subplate is connected to the thalamus through thalamocortical and corticothalamic projections (43, 44). Work by O'Leary and colleagues (45) indicates that functional properties of the cortex require (and may be specified by) these thalamic connections. For example, efferent axons from subplate neurons pioneer pathways from the visual cortex to the internal capsule, thalamus, and other regions of the brain (42, 46). Thus, we speculate that the injury to, or maldevelopment of, the cortical layers in polymicrogyria spares the subplate or develops after functional cortical identity has been established via subplate connections. We further speculate that this sparing of functional connections may underlie the lack of functional recovery in our patients. The recent work of Van Bogaert et al (28), showing normal fluorodeoxyglucose uptake in the polymicrogyric cortex of their patients with bilateral perisylvian polymicrogyria, supports our speculation that the polymicrogyric cortex retains function (and perhaps functional identity). We suggest that studies could be designed using functional MR imaging or magnetic source imaging to confirm this speculation.
Conclusion
Bilateral symmetrical polymicrogyria seems to develop in specific topological locations within the brain (frontal, perisylvian, medial parieto-occipital, and lateral parietal) and in combinations of those regions. Affected patients seem to manifest reasonably well-defined symptom complexes that correlate with the regions of the cortex that are involved. Although evidence in the literature suggests a vascular cause for polymicrogyria, a number of reported familial cases raise the possibility of a genetic cause or genetic susceptibility.
Footnotes
↵1 Address reprint requests to A. James Barkovich, MD, Department of Radiology, Neuroradiology Section L 371, University of California San Francisco, 505 Parnassus Ave, San Francisco, CA 94143.
References
- Received April 12, 1999.
- Copyright © American Society of Neuroradiology