With the advancement of functional imaging and stroke therapy, patients with acute stroke now have a realistic opportunity to benefit from early diagnosis and prompt intervention (1–5). Great enthusiasm and expectation have been generated in stroke imaging, particularly with diffusion-weighted imaging, which has been reported to be highly sensitive and specific in the detection of ischemic changes of parenchyma and in the prediction of infarction in both animal models and in humans (6–25). Although the evaluation of acute ischemia with diffusion-weighted imaging appears to be impeccable, controversies have also arisen (18, 21, 26–30).
Having a better understanding of the underlying pathophysiology of ischemic stroke, we have discovered a vast array of events that can occur after the initial ischemic insult (31–38). Further complicating the issue, all these elements may be involved to various degrees at different times after the onset of the initial ischemic insult. Any attempt to apply a single parameter obtained at a single time point (including diffusion-weighted imaging) to resolve such a dynamic and complex disease process will not be completely successful. Regardless of how complex and dynamic the stroke process can be, we must realize that it is the pre-existing collateral circulation that plays the most critical role to permit us to have an opportunity to treat the stroke (therapeutic window) and to influence the clinical and therapeutic outcome. With complete cessation of blood supply and inadequate collateral circulation, as in cardiac arrest, a neuron will die within 5 minutes. In an occluded artery, re-establishment of blood supply (thrombolysis/recanalization) to ischemic tissue that has been without adequate collateral circulation will not be able to salvage (reverse) the ischemic tissue and may actually cause hemorrhagic complications. In addition, the evolution and outcome of the ischemic injury depends upon the severity and duration of ischemia, which again is critically influenced by collateral circulation (39–42).
Two articles by Lefkowitz et al and Wang et al (pages 1871 and 1876, respectively) in this issue of the AJNR reported that infarction can occur in brain parenchyma while diffusion-weighted imaging findings obtained during hyperacute ischemia are negative. In addition, diffusion-weighted imaging can be negative when perfusion-weighted imaging shows extensive abnormalities. These two articles support the notion that the conclusions made by prior studies need to be re-examined in their full context. We must consider the type of patient population (treated versus untreated) that prior reports derived conclusions from as well as the changing role of stroke imaging within expanded clinical needs, including detection/confirmation of acute strokes and assessment of viability/reversibility of ischemic tissue.
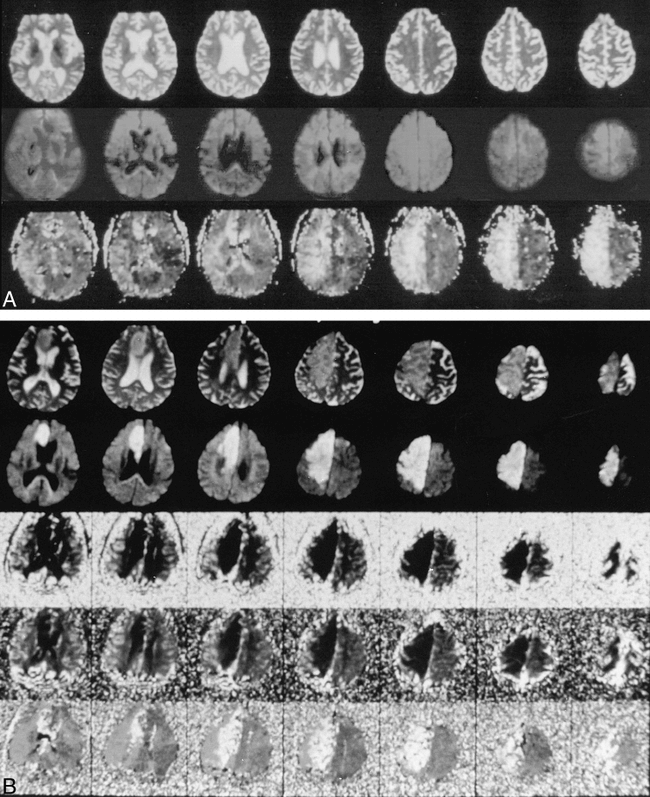
Some images of cases reported by these two articles may, particularly in retrospective review, show diffusion abnormality that could represent early ischemic changes. Some of these diffusion changes were subtle when compared with the corresponding perfusion-weighted imaging findings and might not be easily appreciated prospectively. Even if all these changes found retrospectively were true, one can argue that the majority of the infarcted tissue actually did not have diffusion abnormality on the initial examinations. Therefore, the majority of the infarcted tissue of these cases support the fact that ischemic parenchyma with initial negative diffusion-weighted imaging findings can develop into infarction later (Figs 1–2). In addition, marked perfusion abnormality without diffusion abnormality can occur during acute ischemia, which may or may not evolve into infarction in patients with or without recanalization treatment. These facts are contrary to the general belief that diffusion-weighted imaging is more sensitive than perfusion-weighted imaging in detecting acute ischemia and more specific in prediction of ischemic outcome (infarction).
A, T2-weighted (top), diffusion-weighted (middle), and perfusion-weighted (TTP) (bottom) images obtained 2.5 hours after onset of symptoms. T2 and diffusion findings are negative, but profound perfusion abnormality (hyperintensity) of the right hemisphere is shown.
B, 3-day follow-up T2-weighted (top), diffusion-weighted (middle), and perfusion-weighted (bottom) images (rCBV, max ΔR2*, and TTP) show large right anterior and middle cerebral artery infarctions.
(Courtesy of Michael E. Moseley, Stanford University.)
Before making any judgments about these discrepancies, however, there are fundamental principles that have not been emphasized in the past that deserve to be addressed. These include the complex and dynamic pathophysiology involving acute ischemia, differences in capabilities/limitations between techniques, potential bias by the patient population (treated versus untreated), and the expanding roles of stroke imaging in clinical diagnosis and treatment.
Prior studies did not include patients with early recanalization (treated patients). Based upon the untreated patient population, diffusion-weighted imaging has been reported to have a sensitivity and specificity ranging from 88% to 100% and 95% to 100%, respectively, in the diagnosis of acute stroke and prediction of infarction (9, 10, 21–25). Are we satisfied with these prior reports or have we “over-rated” the efficacy of diffusion-weighted imaging in the diagnosis of acute stroke as judged by the “gold standard” set up by early investigators? Despite the high sensitivity of diffusion-weighted imaging for the detection of ischemia, the clinical or bedside diagnosis of acute stroke by the clinician without the aid of diagnostic imaging has been excellent. Von Arbin et al (43) prospectively evaluated 2252 patients who had been admitted to a medicine department and found that stroke was clinically diagnosed with a sensitivity of 86% and a specificity of 99%. They point out that their bedside diagnosis of stroke could have had a sensitivity of 97% and specificity of 100% had clinical criteria been adhered to more strictly. Several other studies have also analyzed the accuracy of the bedside diagnosis of stroke. In seven such studies (44–50), a total of 2213 patients were given the diagnosis of stroke based on clinical information with a sensitivity of 90.2% (range, 81%–98.5%). Clearly there is variability in the sensitivity of the bedside diagnosis of stroke, but an expert clinician usually can make a correct diagnosis with high accuracy. Therefore, the real contribution of diffusion-weighted imaging thus far has been early confirmation of the clinician's suspicion of acute stroke, particularly within the first 6 hours of symptom onset, in the vast majority of cases.
In addition, the capability of predicting infarction size has been reported to be different with different diffusion-weighted imaging techniques (20, 26, 51). Furthermore, prior reports suggested that ischemic penumbra (reversible ischemia) can be assessed by perfusion-, or diffusion-weighted imaging, or both, based upon untreated patients (24, 52, 53). Nonetheless, the definition of penumbra is ischemic tissue at risk that is potentially reversible (reversibility) only if early recanalization occurs (ie, in treated patients). Without early and successful treatment, the penumbra will become infarcted and therefore cannot be adequately assessed in the untreated patient population, as was done in prior reports. The penumbra cannot be differentiated from the dead (nonviable) and irreversibly damaged tissues by comparing the perfusion-weighted imaging findings obtained during acute ischemia with the follow-up study. Despite that diffusion-weighted imaging has high sensitivity and specificity for acute stroke in the untreated patient, its efficacy in the assessment of tissue viability and reversibility, particularly for early intervention, has not been established (5, 18, 26, 54–56). Diffusion-weighted imaging has been reported to overestimate (Figs 2–3) (18, 55, 57, 58) or underestimate infarction (Figs 1–2) (21, 24, 51), as demonstrated in the two articles by Wang et al and Lefkowitz et al. In addition, reversal of the diffusion abnormality has been reported after thrombolysis of acute ischemic stroke (54).
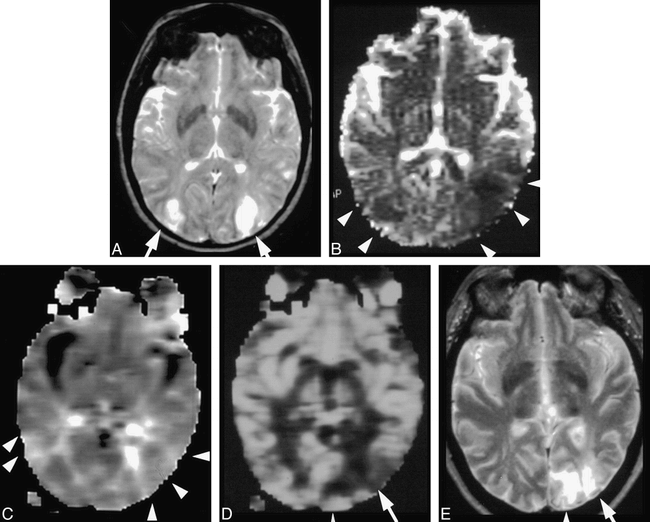
72 hours after the onset of stroke symptoms in a patient with vasculitis.
A, T2-weighted MR image shows bilateral posterior watershed lesions (arrows).
B, The ADC map also shows bilateral posterior watershed lesions (arrowheads), but both lesions are larger than those seen on T2-weighted images (A).
C, The rMTT map shows hypoperfusion (hyperintensity) of both posterior watershed territories (arrowheads).
D, The rCBV map shows only a left-sided lesion (arrow), including posterior watershed and part of the posterior cerebral artery territory (arrowhead).
E, The 16-month follow-up T2-weighted image confirmed small left posterior watershed infarction (arrow) and new infarction at the territory of the left posterior cerebral artery (arrowhead), which was not apparent on initial T2-weighted image (A) or ADC map (B) but was partially visible on rCBV map (D). The abnormality of the right posterior watershed area initially demonstrated on the T2-weighted image (A) and ADC map (B) did not develop into infarction (E). It was accurately predicted by the rCBV map whereas the T2-weighted image and ADC map were falsely positive. The left posterior cerebral artery infarction (arrowhead) was partially diagnosed by the rCBV map, but the ADC map and T2-weighted images were falsely negative.
(Permission granted from the AJNR 20:983–989, June/July 1999.)
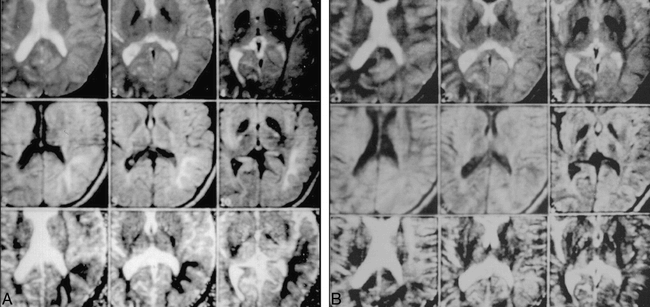
A, T2-weighted (top) and diffusion-weighted (middle) as well as ADC map (bottom) obtained 11 hours after onset of symptoms show normal T2 and abnormal diffusion (hyperintensity). ADC findings (hypointensity) are in the left parietal lobe of the ADC map.
B, T2-weighted (top) and diffusion-weighted images (middle) and ADC map (bottom) obtained 35 hours after onset of symptoms show disappearance of the abnormality previously noted on diffusion-weighted image and ADC map obtained at 11 hours (A) and persistent normal T2. These imaging findings are consistent with the resolution of clinical symptoms.
(Courtesy of Michael E. Moseley, Stanford University.)
Acute ischemia involves rather complex and dynamic pathophysiologic processes. For imaging purposes, it can be divided into three sequential (time-dependent) pathophysiologic processes. The imaging findings depend upon the time of onset as well as the predominant underlying pathophysiology when the imaging is obtained. The temporal changes in the underlying pathophysiology of acute cerebral ischemia can be generalized into three sequential stages: 1) flow abnormalities; 2) cellular dysfunction; and 3) structural breakdown (19–20, 36, 37). Flow abnormality is a kinetic phenomenon that can be detected immediately (19, 20). Perfusion-weighted imaging, therefore, is likely to provide the earliest and most direct imaging information about the regional cerebral blood flow (CBF) that causes ischemia even at the microcirculatory level. As time proceeds, the diminished CBF results in cellular dysfunction as a result of Na+-ATP pump failure. This causes abnormal water shifts (diffusion abnormality) and abnormal accumulations of lactate (spectroscopic abnormality). Therefore, both diffusion-weighted imaging and spectroscopy provide early biological signs of ischemia indirectly, reflecting the underlying flow abnormality, and may not be specific for ischemia (59–60). These changes require time (ie, usually a few hours after onset of symptoms) to accumulate sufficient changes to be appreciated by imaging. This, in part, may explain initial negative diffusion findings and positive perfusion findings in both Lefkowitz et al's and Wang et al's articles. Finally, blood brain–barrier breakdown, a structural disruption, occurs approximately 6 hours after onset of ischemia and allows intravascular content to extravasate into the extracelluar space, which then can be detected by T2-weighted fluid-attenuated inversion-recovery imaging (19–20).
The question of what has contributed to diffusion abnormalities has been long debated. Most popular hypotheses involve “abnormal water movement” related to “cytotoxic edema” (61–66). Although many studies that have investigated where these water molecules circulate and how much cytotoxic edema is involved, definitive proof for the basis of the diffusion abnormality has not yet been established. Currently, there has been more work done on evaluating water molecule movement and cytotoxic edema during acute stroke than on obtaining crucial information regarding the status of the collateral circulation for acute patient management. Nevertheless, the status of tissue blood flow (ie, the severity and duration of ischemia), the fundamental underlying pathophysiologic process influenced by collateral circulation, is more important in the microenvironmental emergency management of patients than the oft-cited “cytotoxic edema” and is more influential for the treatment outcome in acute stroke.
In contrast to most beliefs, diffusion-weighted imaging can provide false-negative (Figs 1–2) or false-positive (Figs 2–3) information regarding infarction because of the complex and dynamic process of acute stroke, heterogeneity of imaging techniques (such as different b-values), and sensitivity to hypoperfusion (18, 21, 26–30, 57, 58). Different imaging techniques provide different types of information reflecting the CBF in the previously mentioned three sequential pathophysiologic processes, and have different sensitivities to degree of hypoperfusion. CBF of normal brain parenchyma ranges from 45–110 cc/100gm/min, and varies with time and location in the same individual. Within the range of hypoperfusion, it includes oligoemia (in asymptomatic patients) and ischemia (in symptomatic patients) (67–68). The CBF threshold of ischemia (ischemic threshold) is generally believed to be around 20 cc/100 gm/min. The ischemic parenchyma is believed to suffer from infarctions quickly if the CBF is below 10 cc/100 gm/min (infarction threshold). CBF between 10–20 cc/100 gm/min is generally considered reversible ischemia, and parenchyma with CBF in this range is called penumbra.
The abnormal findings demonstrated by diffusion-weighted imaging and spectroscopy have been reported to be associated with a CBF threshold above the ischemic range (ie, oligoemia). In one animal study of diffusion MR imaging in global ischemia, Busza et al (7) reported a slight increase in signal at 20 < CBF < 30 mL/100 g/min and a sharp increase in signal at CBF < 15–20 mL/100 g/min. Although the apparent diffusion coefficient (ADC) value is sensitive to change in CBF, it is a function of a number of variables, of which CBF is one. Kohno et al (8) reported regional correspondence of hyperintensity in diffusion-weighted imaging, with perfusion deficits at CBF thresholds of 34 mL/100 g/min after 30 minutes and 41 mL/100 g/min after 2 hours of middle cerebral artery occlusion. Their reports suggest that the threshold for ADC changes at a given CBF depends on the duration of ischemia. Therefore, abnormalities revealed by these imaging techniques are not always specific for ischemia or indicative of infarction (5, 18, 54, 56–60).
In conclusion, Wang and colleagues and Lefkowitz and colleagues amplify the need for re-examination of the capabilities and limitations of new techniques, including those of diffusion-weighted imaging. The analysis should include not only untreated but also treated patients, particularly in the assessment of viability and reversibility. Diffusion- and perfusion-weighted imaging should be used simultaneously and interpreted with caution for the expanding clinical needs of stroke imaging. The future holds real excitement and challenges us to provide valuable information to salvage ischemic tissue promptly.
Acknowledgments
I would like to express my special thanks for the expert input from Drs. Michael E. Mosley, Stanford University, and Joan E. Maley, University of Iowa.
Footnotes
↵1 Address reprint requests to William T.C. Yuh, MD, Department of Radiology, University of Iowa Hospital, 0416 JCP, Iowa City, Iowa 52242.
References
- Copyright © American Society of Neuroradiology